Method for preparing liquid type PT agent based on human recombinant thrombokinase
A technology of thromboplastin and human recombination, which is applied in the biological field, can solve problems such as the inconvenient process, and achieve the effects of avoiding changes in measured values, complete protein molecular structure, and easy use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Construction and expression of recombinant plasmids
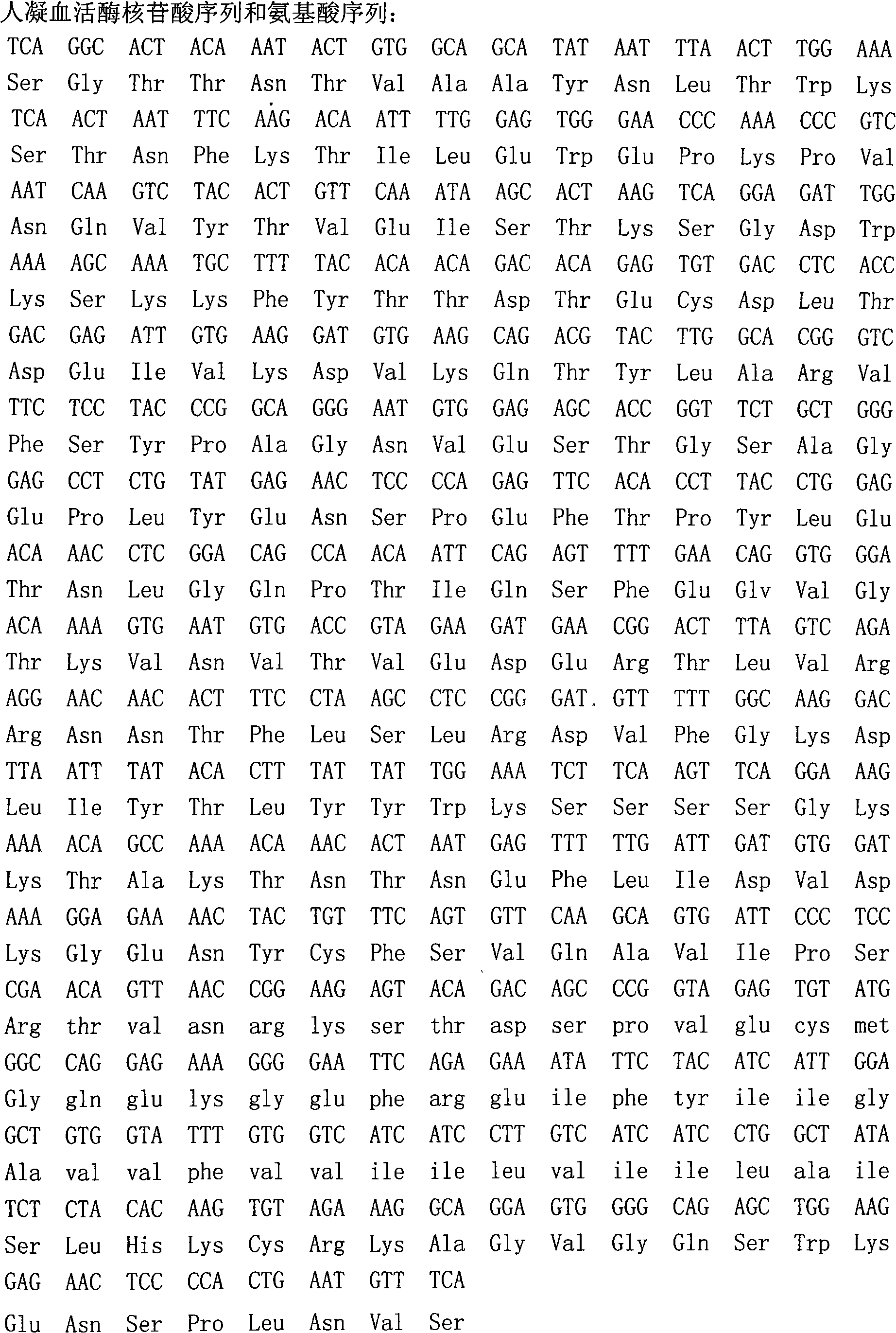
[0019] Total RNA was obtained from human placenta tissue, according to the nucleotide sequence of coagulation factor III in GeneBank ( figure 1 ) Design PCR upstream primer MF3 and downstream primer MR2:
[0020] MF3: 5`-CCGCTCGAGAAAAGATCAGGCACTACAAATACTGCGGC-3`
[0021] MR2: 5`-GGCCTAGGTCAATGATGATGATGATGATGTGAAACATTCAGTGGGGA-3`
[0022] The MF3 primer contains several amino acids at the C-terminal of human thromboplastin, the Xhol cleavage protection group and the Xhol cleavage site, and the MR2 contains several amino acids at the N-terminal of human thromboplastin, the Avr II cleavage site, the terminator codon and 6 His codons son.
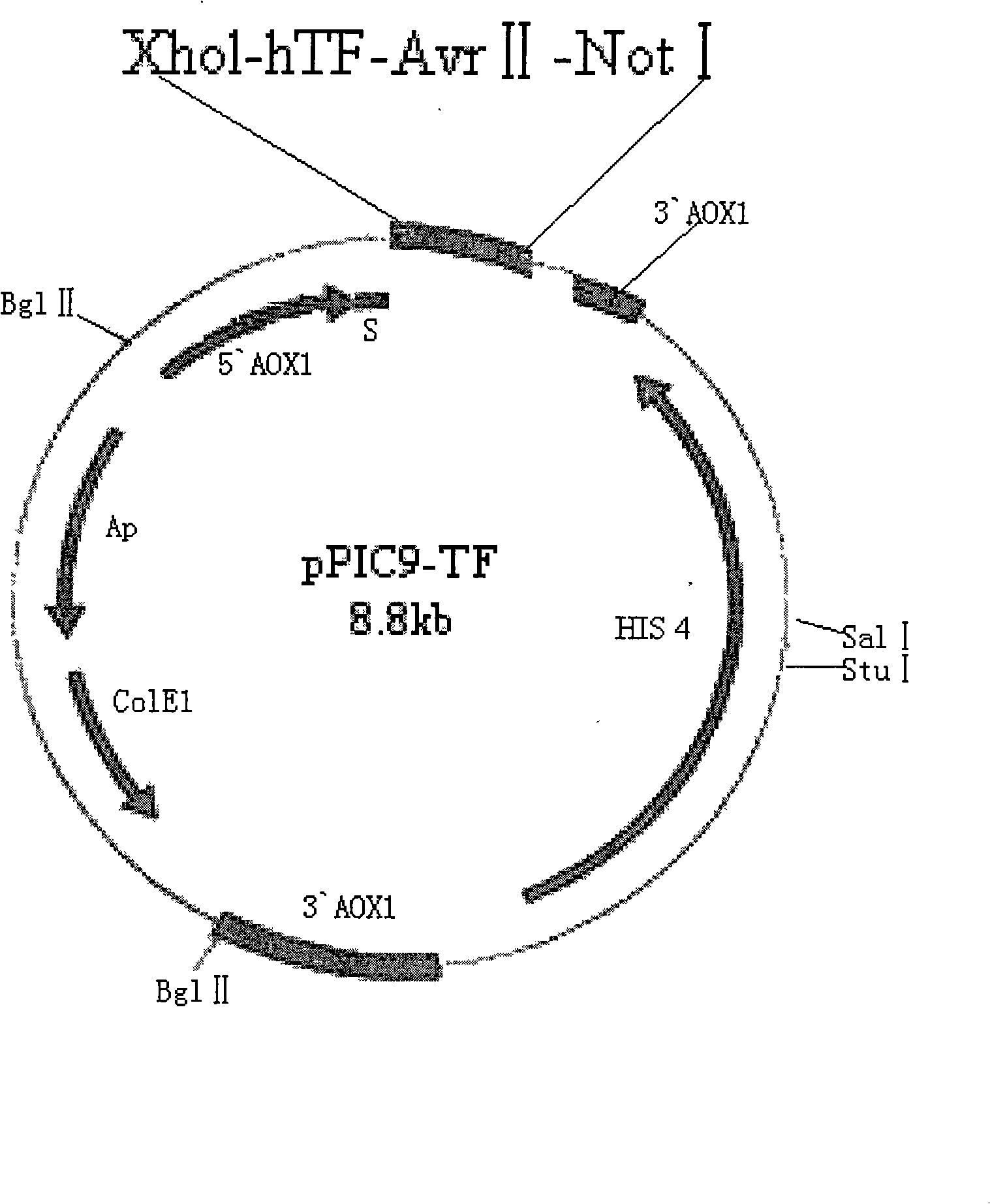
[0023] Using total RNA as a template, human thromboplastin gene was obtained by RT-PCR method. This gene was treated with the above two endonucleases, and then connected to the plasmid pPIC9 to obtain the recombinant expression plasmid ( figure 2 ).
[0024] Transform the recom...
Embodiment 2
[0026] Purification of human thromboplastin
[0027]The selected high-expressing strains were cultivated by conventional methods, and 3% methanol was added at 48 hours to induce foreign proteins. In order to avoid the influence of culture medium components on the purification steps and simplify the purification process, the foreign protein is designed to be expressed intracellularly. The bacterial cells were collected by centrifugation, and the residual culture solution was washed with pH7.4 50mmol / L sodium phosphate buffer solution containing 5% glycerol and 1mmol / L PMSF. The cells were homogenized under high pressure, and then the supernatant containing the target protein was collected by centrifugation, and the target protein was directly purified by Ni-NTA Agarose column chromatography. The equilibrium solution used is pH 7.4 0.02mol / L sodium phosphate buffer (BufferB) containing 0.5mol / L NaCl, which is eluted step by step. Protein, and finally eluted with Buffer B conta...
Embodiment 3
[0029] Preparation of PT kit
[0030] 1. The composition of PT kit: phospholipidated human thromboplastin (1%), buffer and stabilizer (PTF-G).
[0031] 2. PTF-G formula: 40mmol / L Tris-HCl, pH7.0
[0032] Glucose gum, final concentration 4%
[0033] Polyethylene glycol, final concentration 0.5%
[0034] CaCl 2 , final concentration 11mmol / L,
[0035] NaCl, final concentration 30mmol / L,
[0036] Mannitol, final concentration 2%
[0037] Sodium azide, final concentration 0.1%
[0038] Gelatin, final concentration 0.25%
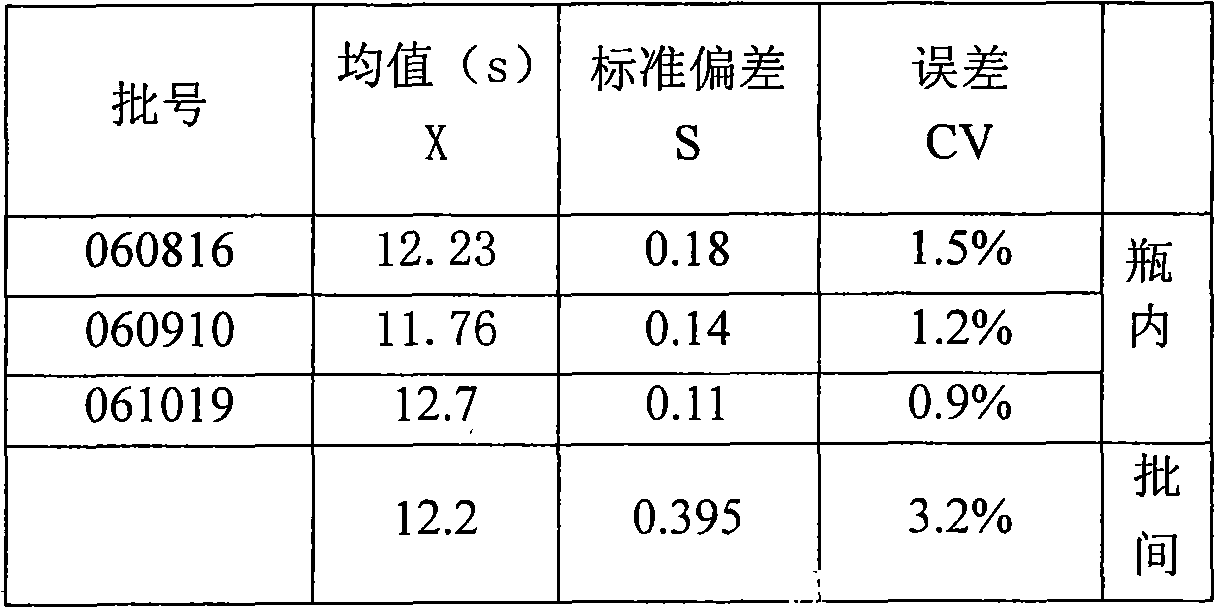
[0039] 3. Detection method: PT reagent is preheated at 37°C for 20 minutes, take 100 μl, incubate the plasma to be tested at 37°C for 3 minutes, take 50 μl, mix well and time, use the CA-530 hemagglutination instrument of Sysmex Company to measure, and record the plasma clotting time .
[0040] 4. Product technical indicators:
[0041] 1) Appearance: Colorless transparent liqui...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com