Methods, compositions and kits for reducing tissue adhesions
A composition and technology of the next group, applied in the direction of drug combination, surgical adhesive, biochemical equipment and method, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0102] Methods of preparing compositions according to some embodiments of the invention
[0103] In some embodiments, the present invention provides a method for obtaining a composition according to some embodiments of the present invention, comprising:
[0104] obtain a plasma sample,
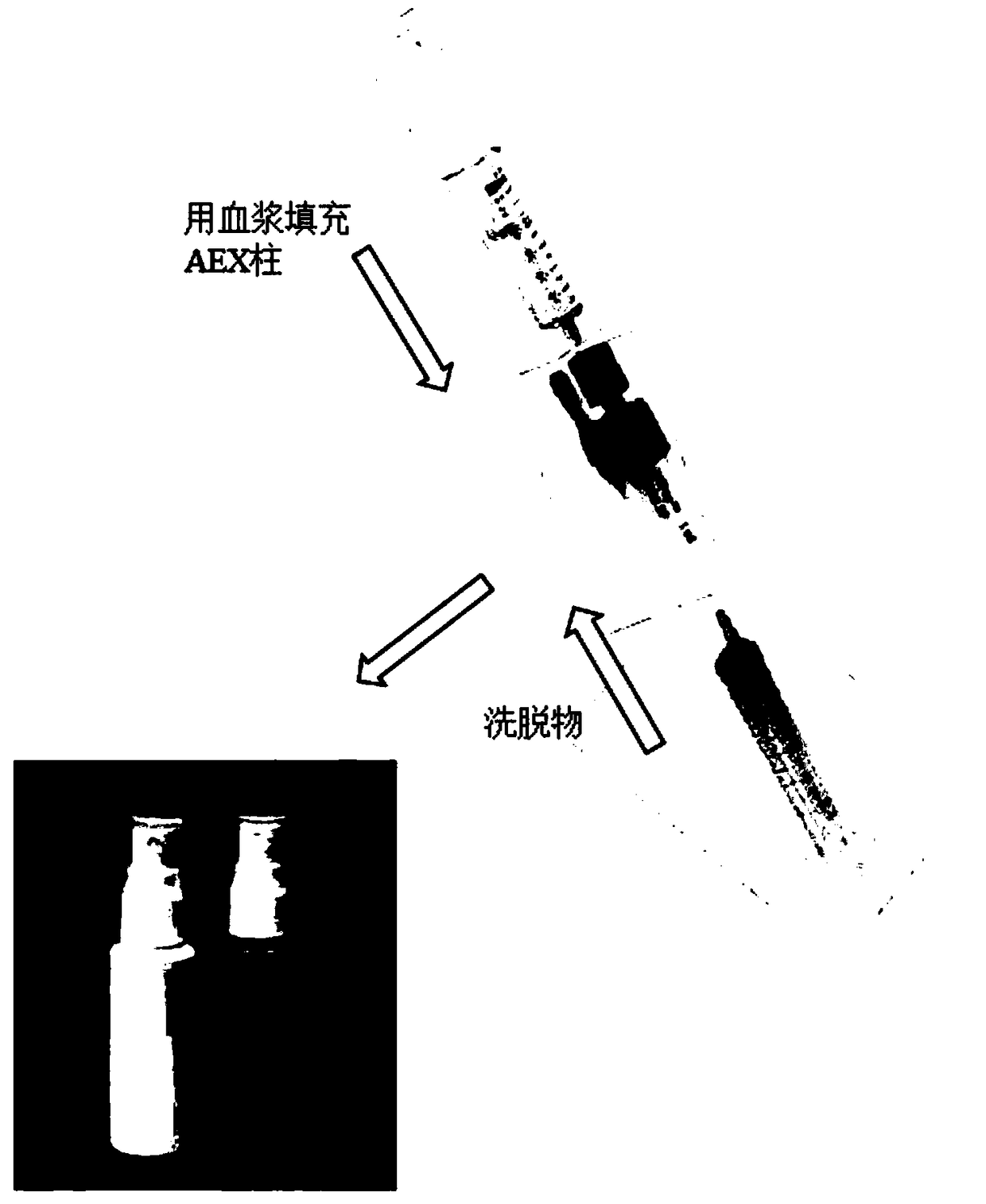
[0105] generating the bound fraction by packing a plasma sample onto an anion exchange column, and
[0106] Compositions according to some embodiments of the invention are obtained by eluting the binding moiety with an eluent.
[0107] In some embodiments, the plasma sample is obtained from a source selected from the group consisting of a subject undergoing surgery, a donor, and a commercially available source. In some embodiments, the plasma sample is between 5 mL and 500 mL.
[0108] In some embodiments, the composition comprises prothrombin (Factor V) and at least one factor selected from the group consisting of Prothrombin (Factor II), Prothrombin (Factor VII), and Stuart - Prower Fact...
Embodiment 1
[0253] Example 1: Preparation of a composition comprising at least one coagulation factor
[0254] Overview:
[0255] First, mammals (such as but not limited to cows, pigs, horses, etc.) are bled, and 1000-2000ml of blood is collected to prepare for purification and concentration of the composition according to some embodiments of the present invention. Blood was centrifuged at 3000 RPM (approximately 1700 RCF) for 10 to 15 minutes to prepare plasma. Plasma is collected, filtered, and passed through an AEX column or other manifold to concentrate negatively charged blood factors. Functional testing was performed for remaining levels of factors II, VII, X and the related cofactor prothrombinogen (V).
[0256] In another embodiment, human plasma is used to prepare a composition comprising at least Factor V.
[0257] In another embodiment, fibrinogen-depleted ESCs are prepared by altering the wash and elution buffers and / or including one or more biocompatible polymers during th...
Embodiment 2
[0292] Example 2: In vivo tests of compositions comprising at least one coagulation factor
[0293] Overview:
[0294] In vivo experiments were performed in rats. However, rats of small size may not be able to draw sufficient blood volumes to operate with the kits presented here (needing >20% blood volume removal). Therefore, human plasma was processed for 5-10 rats (approximately 1 ml per rat, corresponding to approximately 50-100 ml of initial blood for all rats).
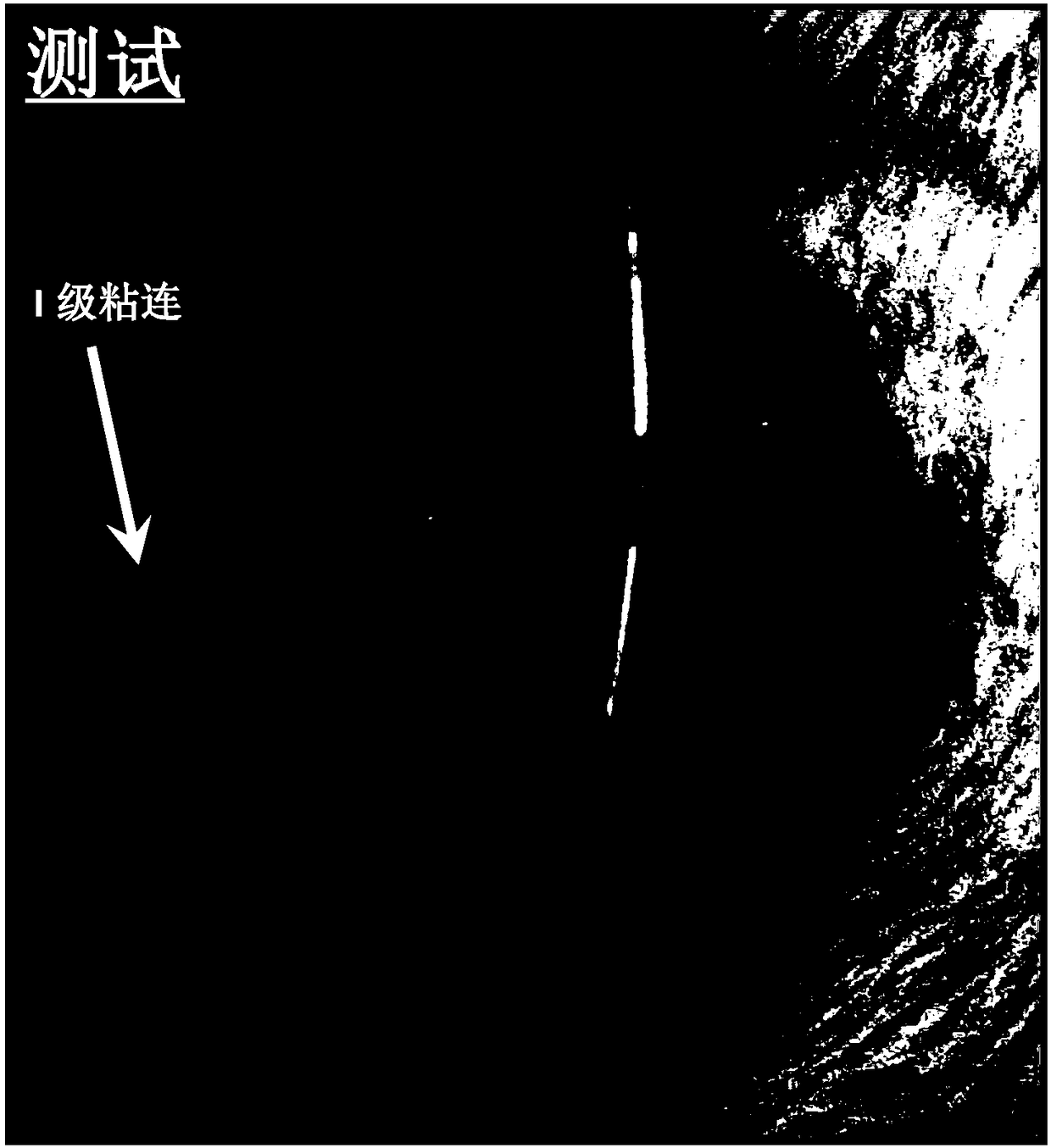
[0295] Rats were anesthetized and the rat peritoneum was exposed for experiments. The adhesion formation procedure (cecal abrasion) was performed in rats. After performing a cecal abrasion, a composition according to some embodiments of the invention is applied to and around the abrasion. Control rats were treated with saline. Two weeks later, animals were sacrificed and adhesion scores were graded blindly. Select representative images for comparison.
[0296] Animal experiment:
[0297] Six (6) adult whi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com