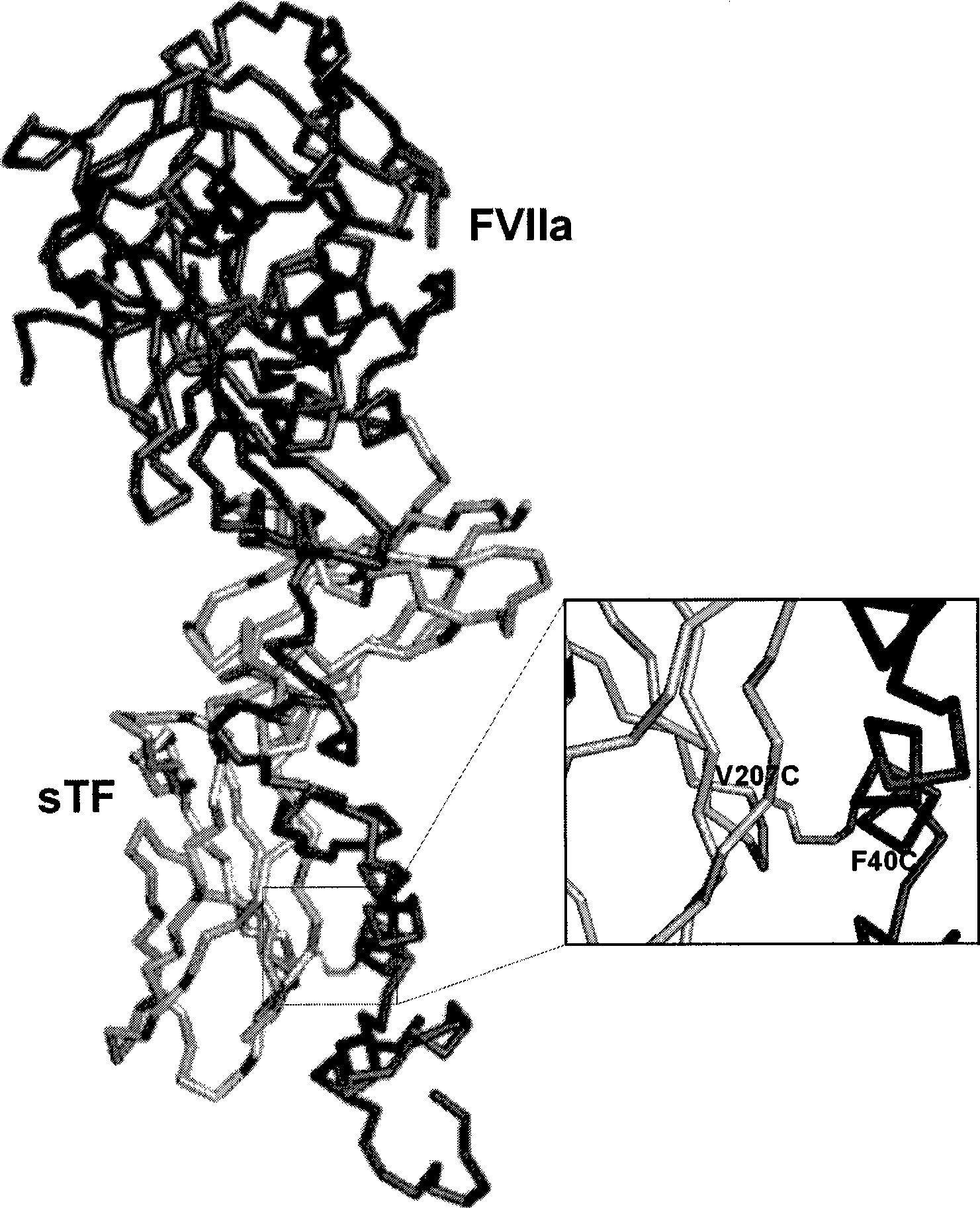
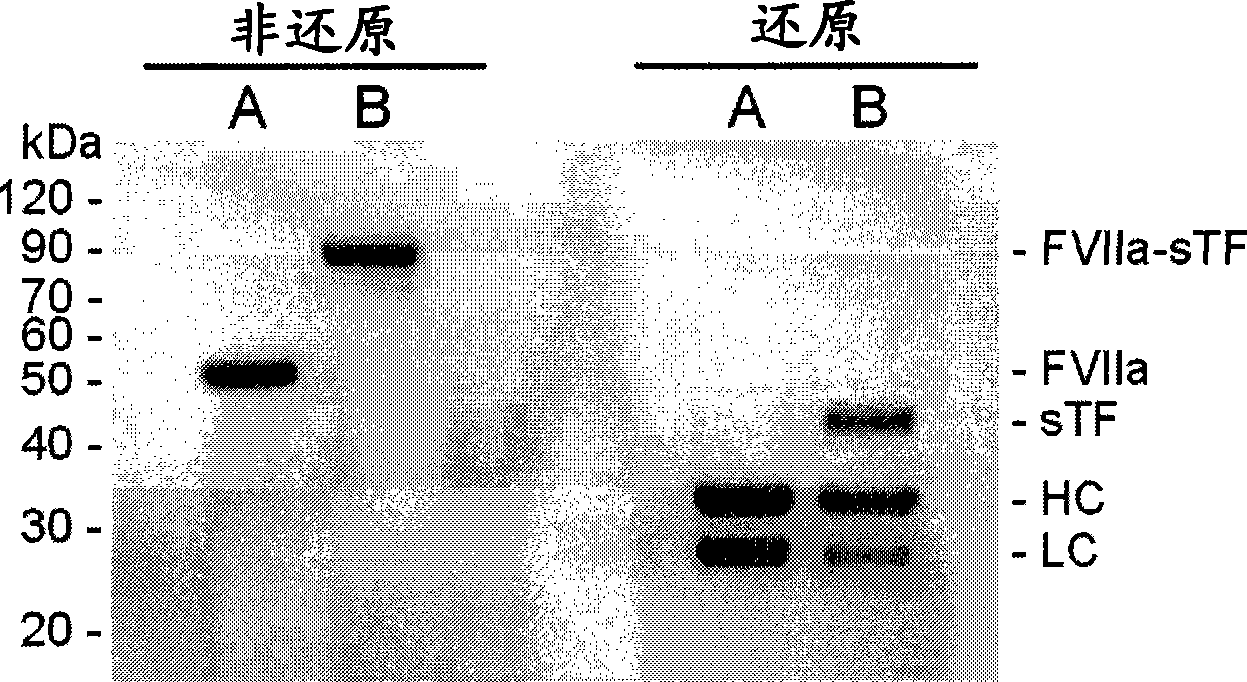
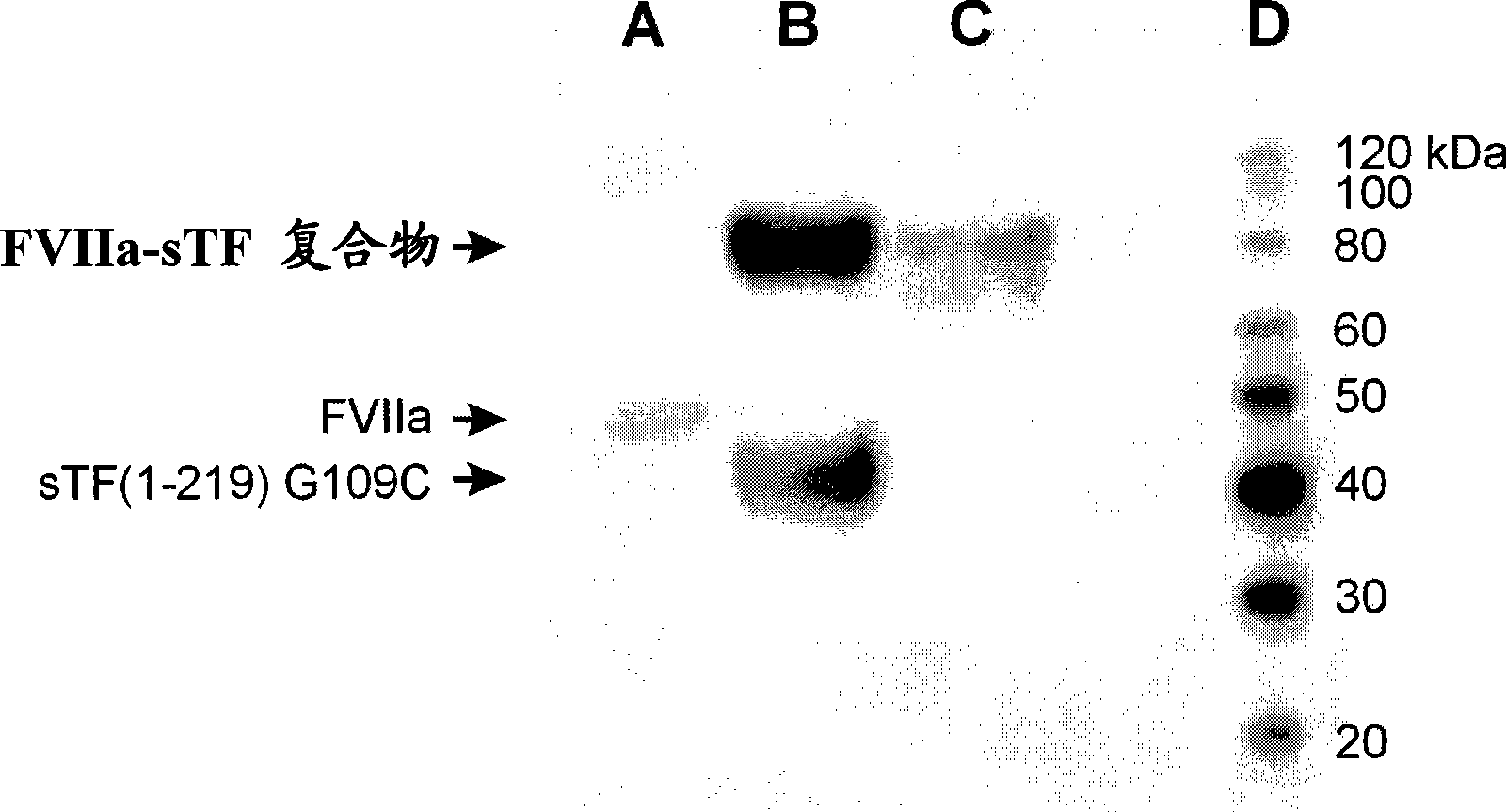
Covalent factor VII-tissue factor complex
A coagulation factor and tissue factor technology, applied in the field of novel covalent complexes, can solve the problem of not showing proteolytic activity of coagulation factor X
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0112] Preparation of complex
[0113] Crosslinking Tissue Factor and Factor VII Using Heterobifunctional Reagents
[0114] In an interesting variant, the method for preparing the complex involves production of cysteine variants of soluble tissue factor followed by labeling of soluble tissue factor with a heterobifunctional reagent in which one functional group is cysteine reactive Cysteine, and finally cross-linked with factor VIIa through the second functional group of the reagent. Methods for cloning and expressing tissue factor cysteine mutants in E. coli and subsequent labeling with cysteine-specific reagents have been previously described (Stone et al. (1995) Biochem. J., 310, 605-614; etc. (1996) Protein Sci., 5, 1521-1540; Owenius et al. (1999) Biophys.J., 77, 2237-2250; et al. (2001) Biochemistry, 40, 9324-9328). Photocrosslinking of proteins using heterobifunctional reagents comprising a cysteine-specific and a photoactivatable functional group has been de...
Embodiment 1
[0128]Materials - D-Phe-Phe-Arg-Chloromethylketone was purchased from Bachem. Chromogenic Z-D-Arg-Gly-Arg-p-nitroaniline (S-2765) and H-D-Ile-Pro-Arg-p-nitroaniline (S-2288) substrates were purchased from Chromogenix (Sweden). Human plasma-derived coagulation factor X (hFX), coagulation factor Xa (hFXa) and coagulation factor IXa (hFIXa) were purchased from EnzymeResearch Laboratories Ltd. (South Bend, IN). Human whole brain Marathon-ready cDNA library was purchased from Clontech (Mountain View, CA). According to published literature ( et al. (1996) Protein Sci., 5, 1531-1540) prepared soluble tissue factor 1-219 (sTF(1-219)) expressed in Escherichia coli. Recombinant Factor VIIa was expressed and purified as previously described (Thim et al. (1988) Biochemistry, 27, 7785-7793; Persson et al. (1996) FEBS Lett., 385, 241-243). All other reagents were of analytical grade or higher grade.
[0129] Construction of DNA encoding coagulation factor VII and coagulation factor VII...
Embodiment 2
[0156] Construction of DNA encoding sTF(1-219)W45C, sTF(1-219)D58C, sTF(1-219)G109C and sTF(1-219)P206C-by Site-directed mutagenesis sTF(1-219) mutants were constructed according to the protocol (Stratagene, La Jolla, CA) using the primer pairs (forward and reverse) listed in Table 3 and pHOJ356 as template. The correctness of all cloned sequences was verified by DNA sequencing.
[0157] Table 3 - Forward and reverse (oligo) primers for TF cDNA site-directed mutagenesis
[0158] Primer mutation Sequence (5'→3') oHOJ172-f W45C CACTAAGTCAGGAGATTGCAAAAGCAAATGCTTTTAC oHOJ172-r W45C GTAAAAGCATTTGCTTTTGCAATCTCCTGACTTAGTG oHOJ173-f D58C CAACAGACACAGAGTGTTGCCTCACCGACGAGATTG oHOJ173-r D58C CAATCTCGTCGGTGAGGCAACACTCTGTGTCTGTTG oHOJ174-f G109C CCTGGAGACAAAACCTCTGCCAGCCAACAATTCAGAG oHOJ174-r G109C CTCTGAATTGTTGGCTGGCAGAGGTTTGTCTCCAGG oHOJ175-f P206C GAAGAGTACAGACAGCTGCGTAGAGTGTATGGGC oHOJ175-r P206C GCC...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com