Process for the preparation of beta-ionylideneacetaldehyde
A technology of fenugreek and acetaldehyde, which is applied in the field of β-galenylidene acetaldehyde, can solve the problems of unsuitable and expensive
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
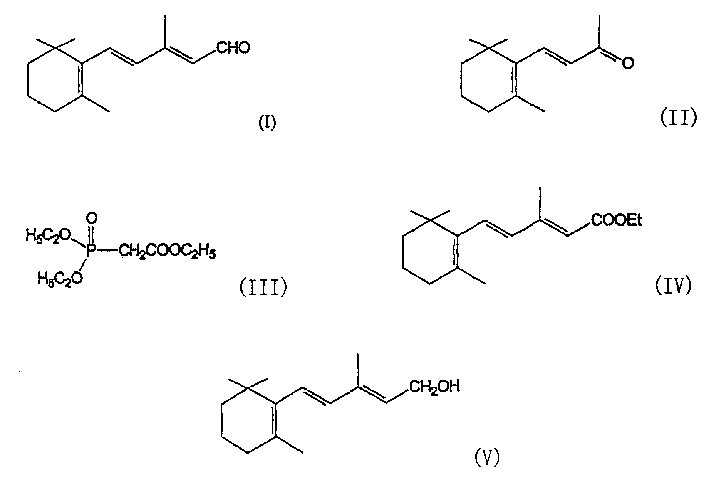
[0035] Preparation of β-zhixiang subunit acetaldehyde (I)
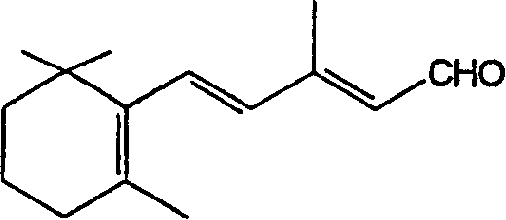
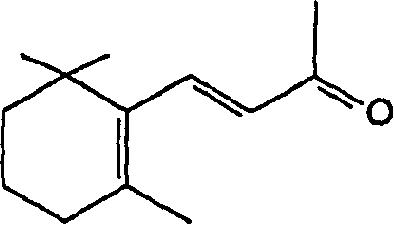
[0036] Step a) Preparation of ethyl β-zhixiangylidene acetate (IV)
[0037] A toluene (1 liter) solution of triethyl phosphonoacetate (1.40 kg) was added to a mixture of sodium amide (0.236 kg) and toluene (6.5 liters) while stirring at about 40°C under nitrogen. The reaction mixture was stirred at 40-45°C for 6 hours, cooled to 0-5°C, and a toluene (1.5 liter) solution of β-genyl and ionone (1 kg) was slowly added at 0-10°C. The reaction mixture was stirred at 65°C for 15 hours and cooled to 20-25°C. Water (4 liters) was added to the reaction mixture and then stirred for another 15 minutes. The toluene layer was separated and distilled under vacuum at 60-80°C to obtain the title compound of formula IV with a yield of 87%, which was a mixture of 9-cis and 9-trans isomers in a ratio of 1:7.
[0038] Step b) Preparation of β-zhixiang subunit ethanol (V)
[0039] Under nitrogen, lithium aluminum hydride (0.11 kg) was added t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com