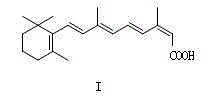
Novel crystal form of isotretinoin as well as preparation method and application thereof
A tretinoin and crystal form technology, applied in the crystal form preparation process and application field of isotretinoin, can solve the problems such as the transformation of isotretinoin crystal form that has never been reported, and achieves overcoming poor stability, The effect of convenient industrial production and easy processing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
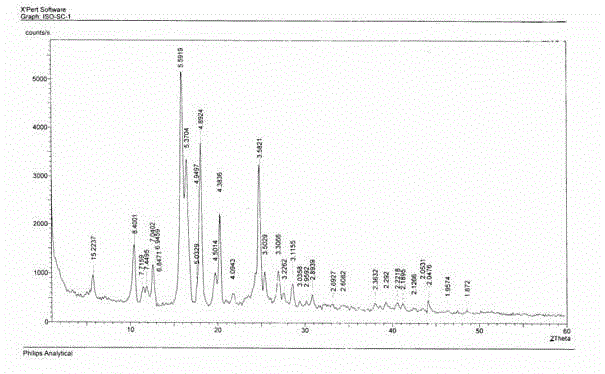
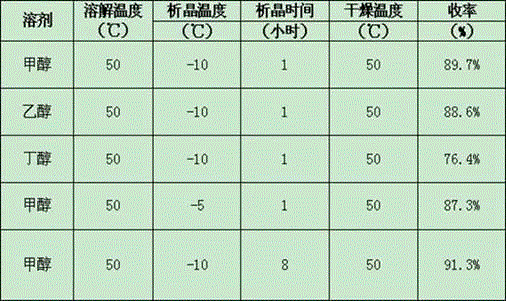
[0043] It is prepared by US4556518 patented technology to obtain the raw material drug of isotretinoin. Under the condition of blowing nitrogen gas, add methanol and the raw material drug of isotretinoin into the reaction bottle, and heat at 50° C. until completely dissolved to obtain an organic alcohol solution of isotretinoin.
[0044] The organic alcohol solution of isotretinoin is crystallized by the temperature difference method, the crystallization temperature is 10°C, the crystallization time is 20 hours, and the solid-liquid separation is carried out by filtration, and the solid is dried at 40°C to obtain isotretinoin A crystal form.
Embodiment 2
[0046] It is prepared by US6177579 patented technology to obtain the raw material drug of isotretinoin. Under the condition of blowing nitrogen gas, add ethanol and the crude drug of isotretinoin into the reaction bottle, and heat at 70° C. until completely dissolved to obtain the organic alcohol solution of isotretinoin.
[0047] The organic alcohol solution of isotretinoin is crystallized by the temperature difference method, the crystallization temperature is -15°C, the crystallization time is 1 hour, and the solid-liquid separation is carried out by filtration, and the solid is dried at 60°C to obtain isotretinoin A Form A of the acid.
Embodiment 3
[0049] It is prepared by US6177579 patented technology to obtain the raw material drug of isotretinoin. Under the condition of blowing nitrogen gas, add butanol and the raw material drug of isotretinoin into the reaction bottle, and heat at 70° C. until completely dissolved to obtain an organic alcohol solution of isotretinoin.
[0050] The organic alcohol solution of isotretinoin is crystallized by the temperature difference method, the crystallization temperature is -15°C, the crystallization time is 1 hour, and the solid-liquid separation is carried out by filtration, and the solid is dried at 60°C to obtain isotretinoin A Form A of the acid.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com