Method for preparing 13-cis isotretinoin
A technology of formic acid and cis-retinoic acid, applied in the field of preparation of 13-cis-retinoic acid, which can solve the problems of cumbersome operation and difficult industrial scale production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
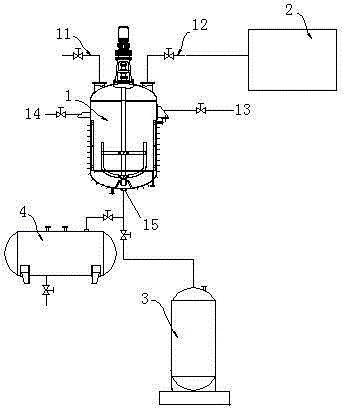
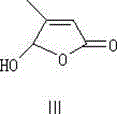
[0064] 372g of [3-methyl-5-(2,6,6-trimethylcyclohexen-1-yl)-2,4-pentadiene]-triphenylphosphine chloride was dissolved in 1000ml of ethanol, Add 84.6g of 5-hydroxy-4-methyl-2-5[H]-furanone under nitrogen, stir until the solution is clear, cool down to -20°C, add 1200ml of 2NKOH ethanol solution dropwise, and keep the temperature at -20± 5°C, react for 2 hours, then adjust the pH value to 7-8 with hydrochloric acid, add 250mg palladium nitrate dissolved in 150ml acetonitrile, heat up to 35°C, detect the reaction result by high performance liquid chromatography (HPLC), and the isomerization reaction is complete Afterwards, pour it into water, add acid to neutralize to pH 2-3, and suction filter to obtain the crude product, which is crystallized with ethyl acetate to obtain 200.2 g of the product, with a purity of 99.7% and a yield of 89.9%.
Embodiment 2
[0066] 372g of [3-methyl-5-(2,6,6-trimethylcyclohexen-1-yl)-2,4-pentadiene]-triphenylphosphine chloride was dissolved in 1000ml of ethanol, Add 84.6g of 5-hydroxy-4-methyl-2-5[H]-furanone under nitrogen, stir until the solution is clear, cool down to -40°C, add 1200ml of 2NKOH ethanol solution dropwise, and keep the temperature at -40± 5°C, react for 2 hours, then adjust the pH value to 7-8 with hydrochloric acid, add 250mg palladium nitrate dissolved in 150ml acetonitrile, raise the temperature to 60°C, detect the reaction result by high performance liquid chromatography (HPLC), and the isomerization reaction is completed Finally, pour it into water, add acid to neutralize to pH2-3, and suction filter to obtain the crude product, which is crystallized with ethyl acetate to obtain 204.7g of the product, with a purity of 99.7% and a yield of 92.1%.
Embodiment 3
[0068] 372g of [3-methyl-5-(2,6,6-trimethylcyclohexen-1-yl)-2,4-pentadiene]-triphenylphosphine chloride was dissolved in 1000ml of ethanol, Add 84.6g of 5-hydroxy-4-methyl-2-5[H]-furanone under nitrogen, stir until the solution is clear, cool down to 15°C, add 1200ml of 2NKOH ethanol solution dropwise, and keep the temperature at 15±5°C , react for 2 hours, then adjust the pH value to 7-8 with hydrochloric acid, add 250 mg of palladium nitrate dissolved in 150 ml of acetonitrile, heat up to 70 ° C, and detect the reaction result by high performance liquid chromatography (HPLC). After the isomerization reaction is completed, Pour it into water, add acid to neutralize to pH 2-3, and filter with suction to obtain the crude product. Crystallize with ethyl acetate to obtain 198.4 g of the product, with a purity of 99.5% and a yield of 88.7%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com