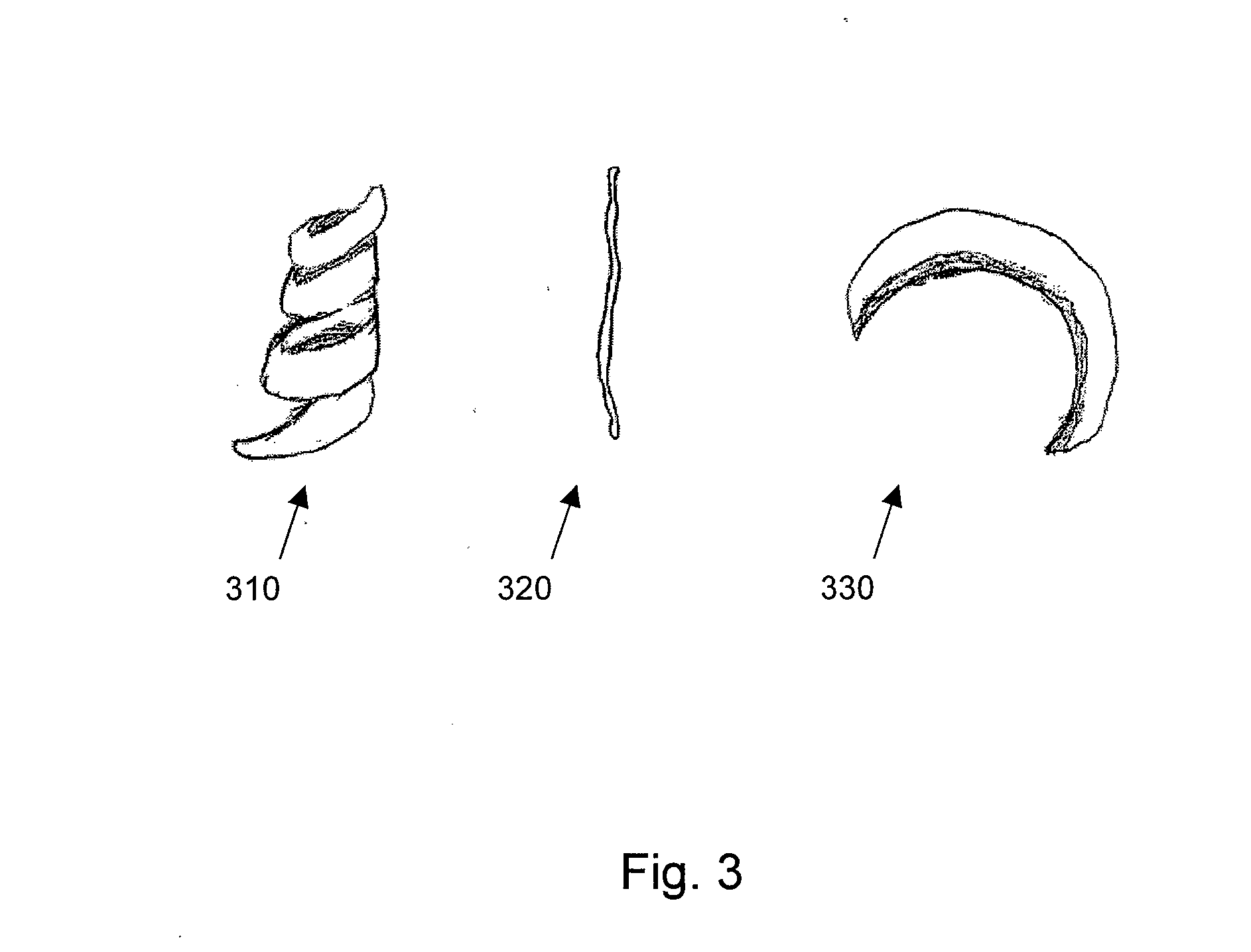
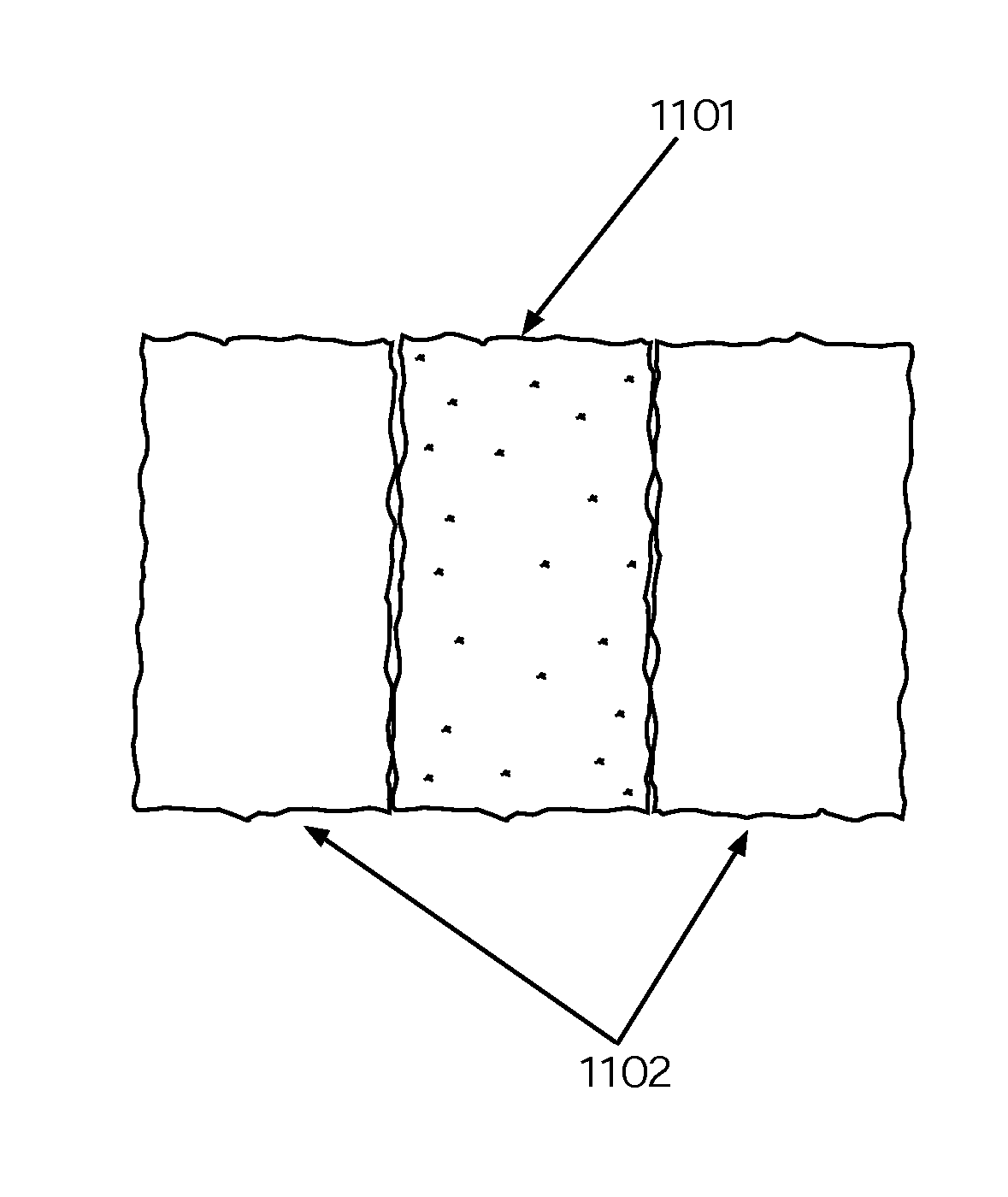
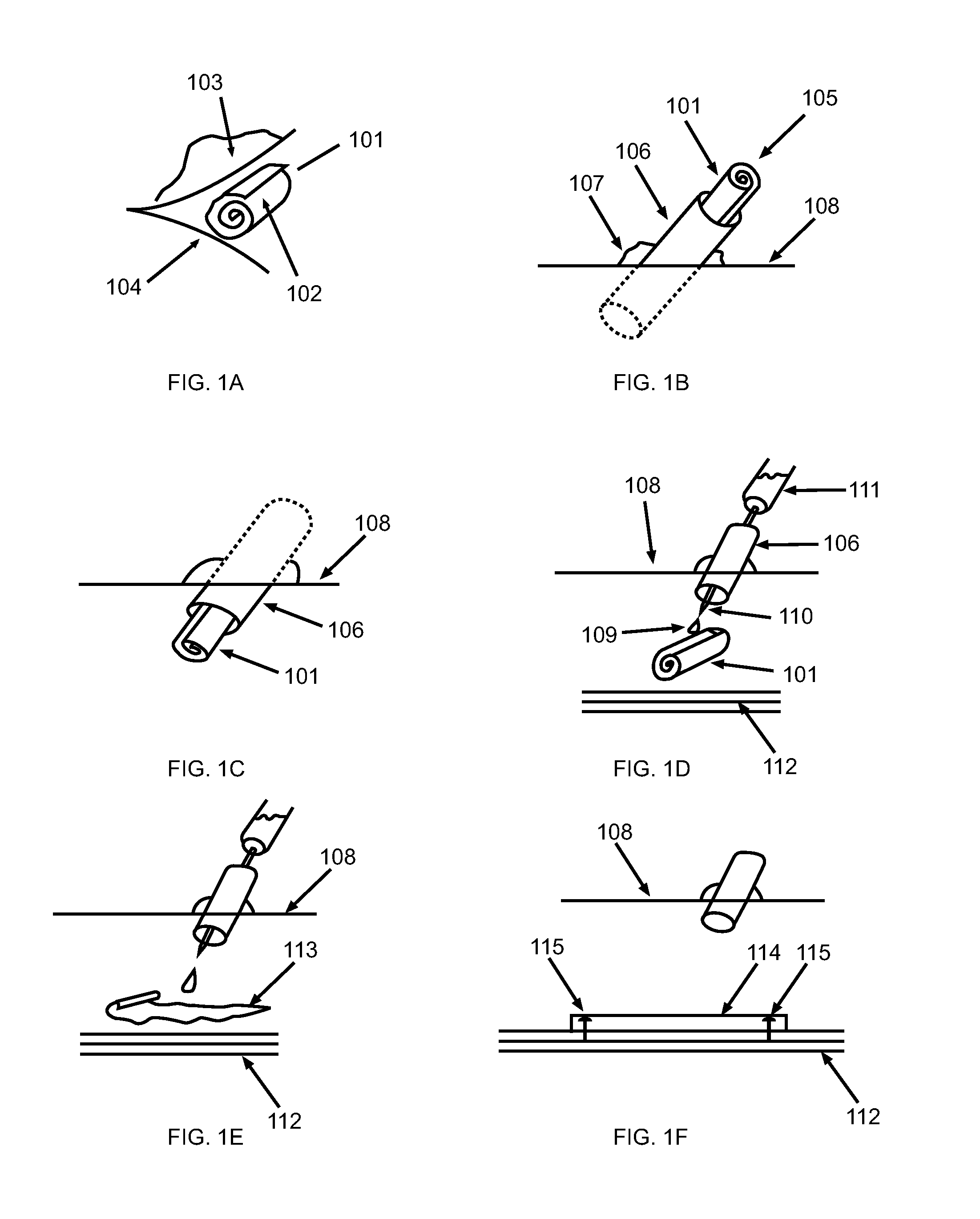
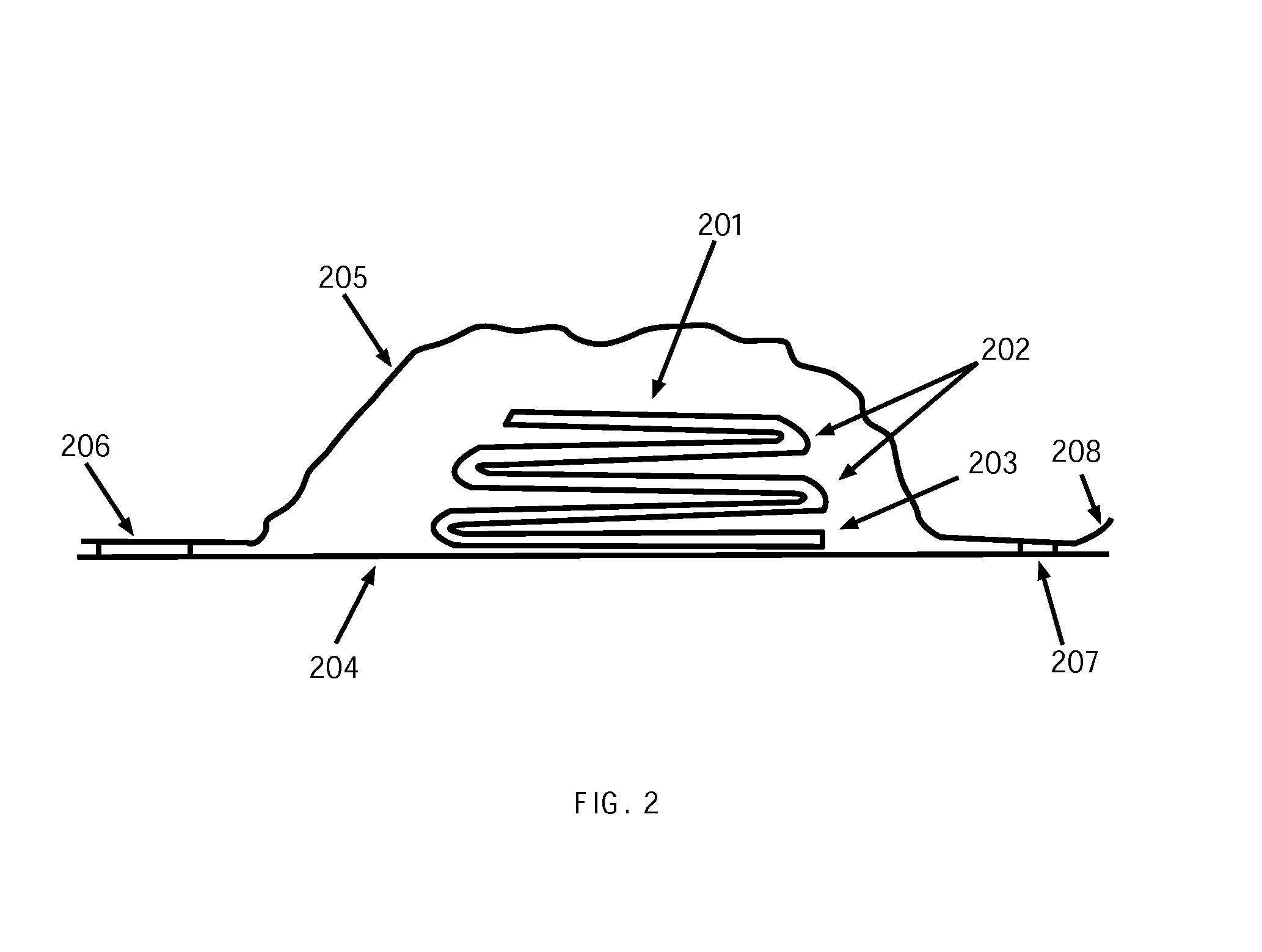
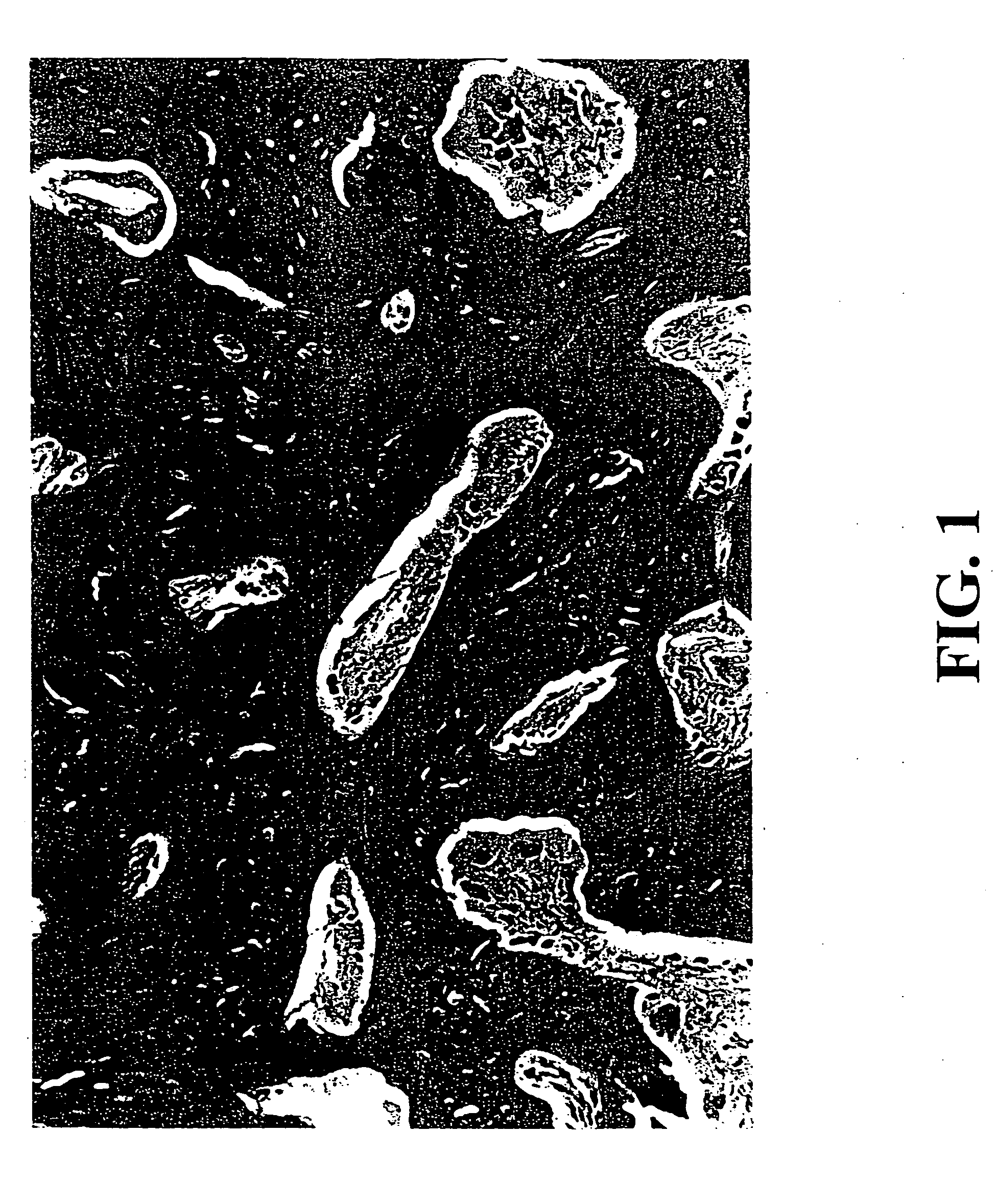
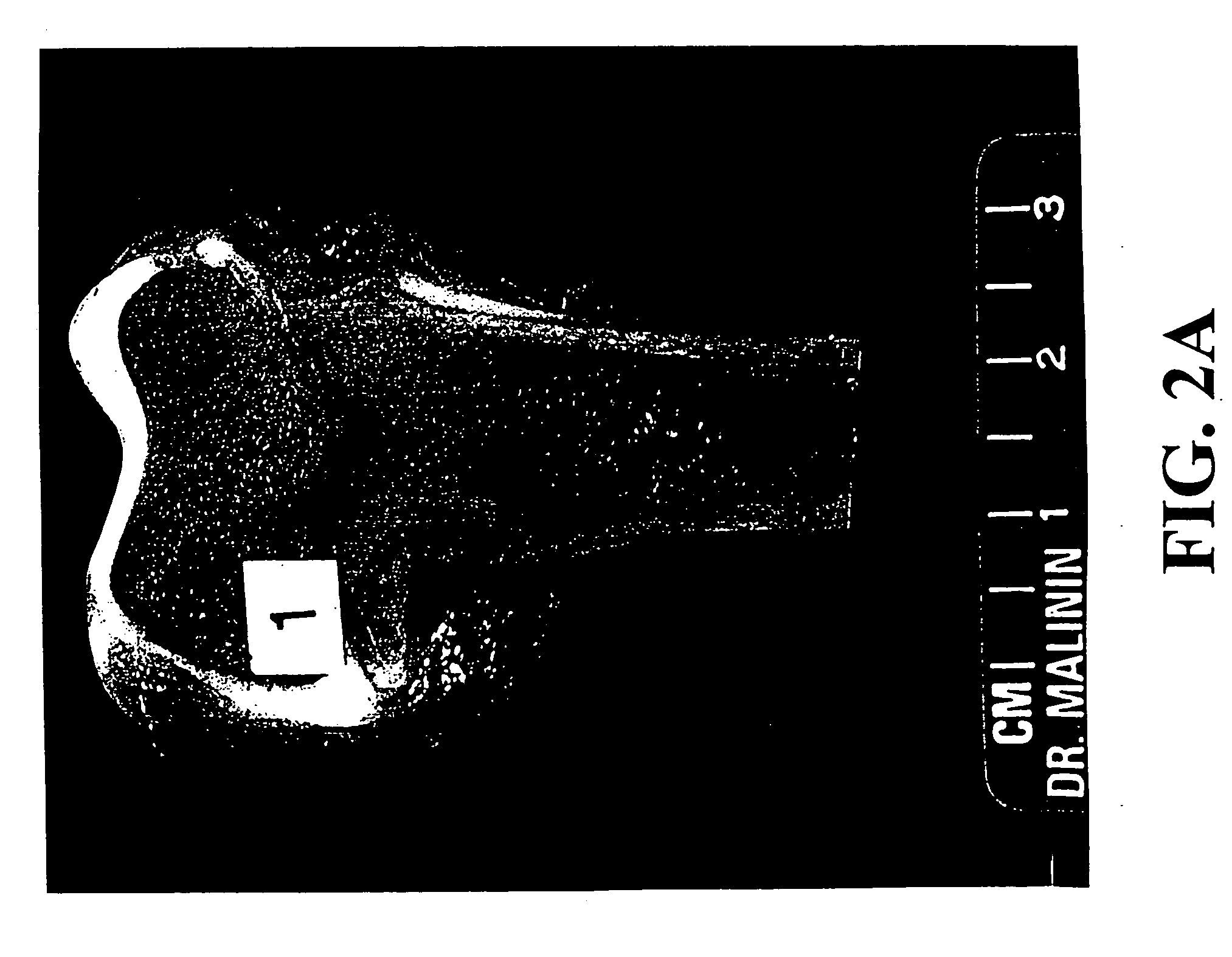
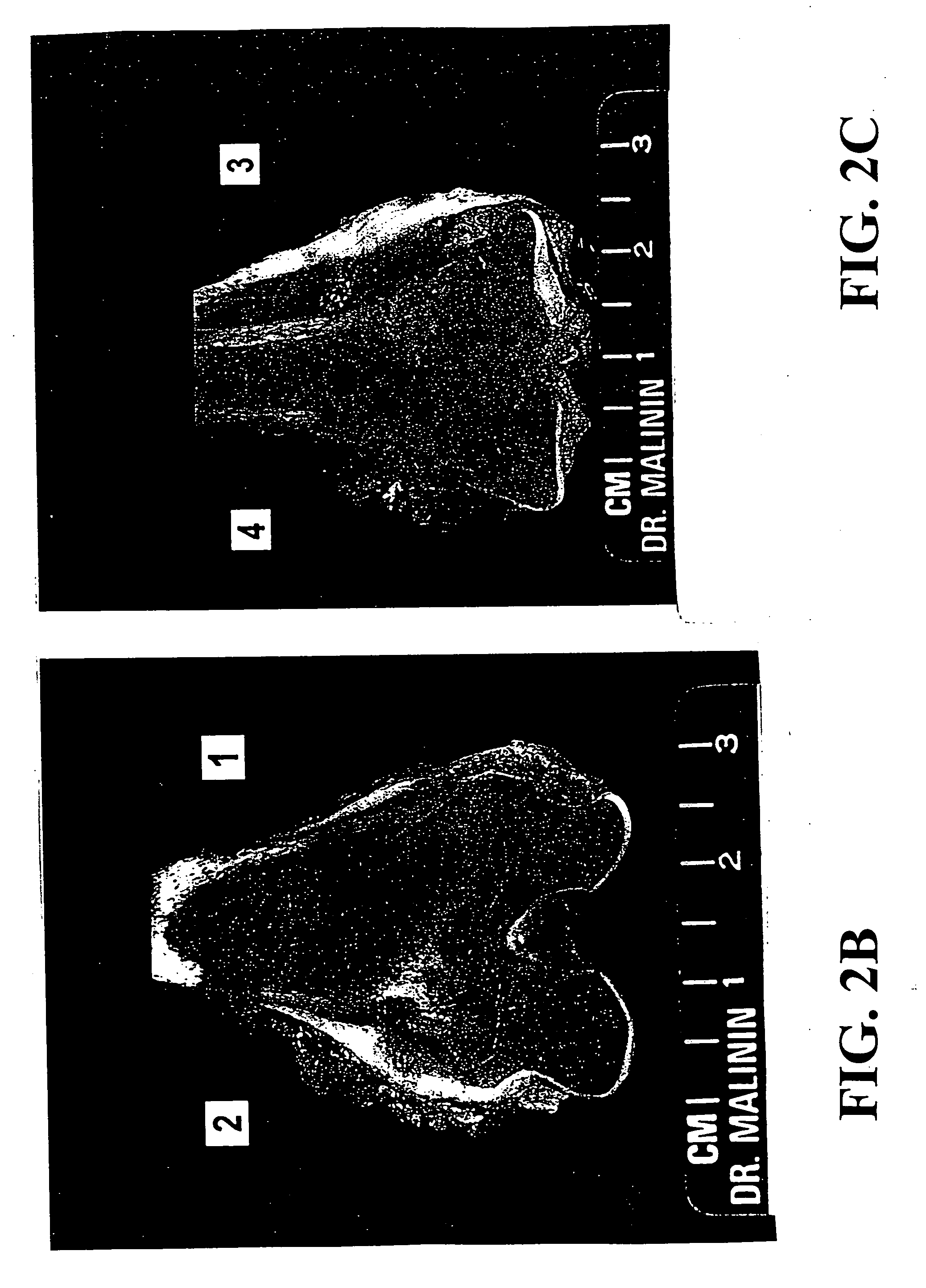
Patents
Literature
125 results about "Demineralized bone matrix" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Demineralized bone matrix (DBM) is allograft bone that has had the inorganic mineral removed, leaving behind the organic "collagen" matrix. It was first discovered by Marshall Urist in 1965 that the removal of the bone mineral exposes more biologically active bone morphogenetic proteins. These growth factors modulate the differentiation of progenitor cells into osteoprogenitor cells, which are responsible for bone and cartilage formation. As a result of the demineralization process, DBM is more biologically active than undemineralized bone grafts; conversely the mechanical properties are significantly diminished.
Bone graft
ActiveUS7163691B2Fast reduction in osteoinductiveGood curative effectOrganic active ingredientsImpression capsOSTEOINDUCTIVE FACTORIn vivo
An improved demineralized bone matrix (DBM) or other matrix composition is provided that has been mixed with a stabilizing agent that acts as (1) a diffusion barrier, (2) a enzyme inhibitor, (3) a competitive substrate, or (4) a masking moiety. A diffusion barrier acts as a barrier so as to protect the osteoinductive factors found in DBM from being degraded by proteolytic and glycolytic enzymes at the implantation site. Stabilizing agents may be any biodegradable material such as starches, modified starches, cellulose, dextran, polymers, proteins, and collagen. As the stabilizing agents degrades or dissolves in vivo, the osteoinductive factors such as TGF-β, BMP, and IGF are activated or exposed, and the activated factors work to recruit cells from the preivascular space to the site of injury and to cause differentiation into bone-forming cells. The invention also provides methods of preparing, testing, and using the inventive improved osteodinductive matrix compositions.
Owner:WARSAW ORTHOPEDIC INC
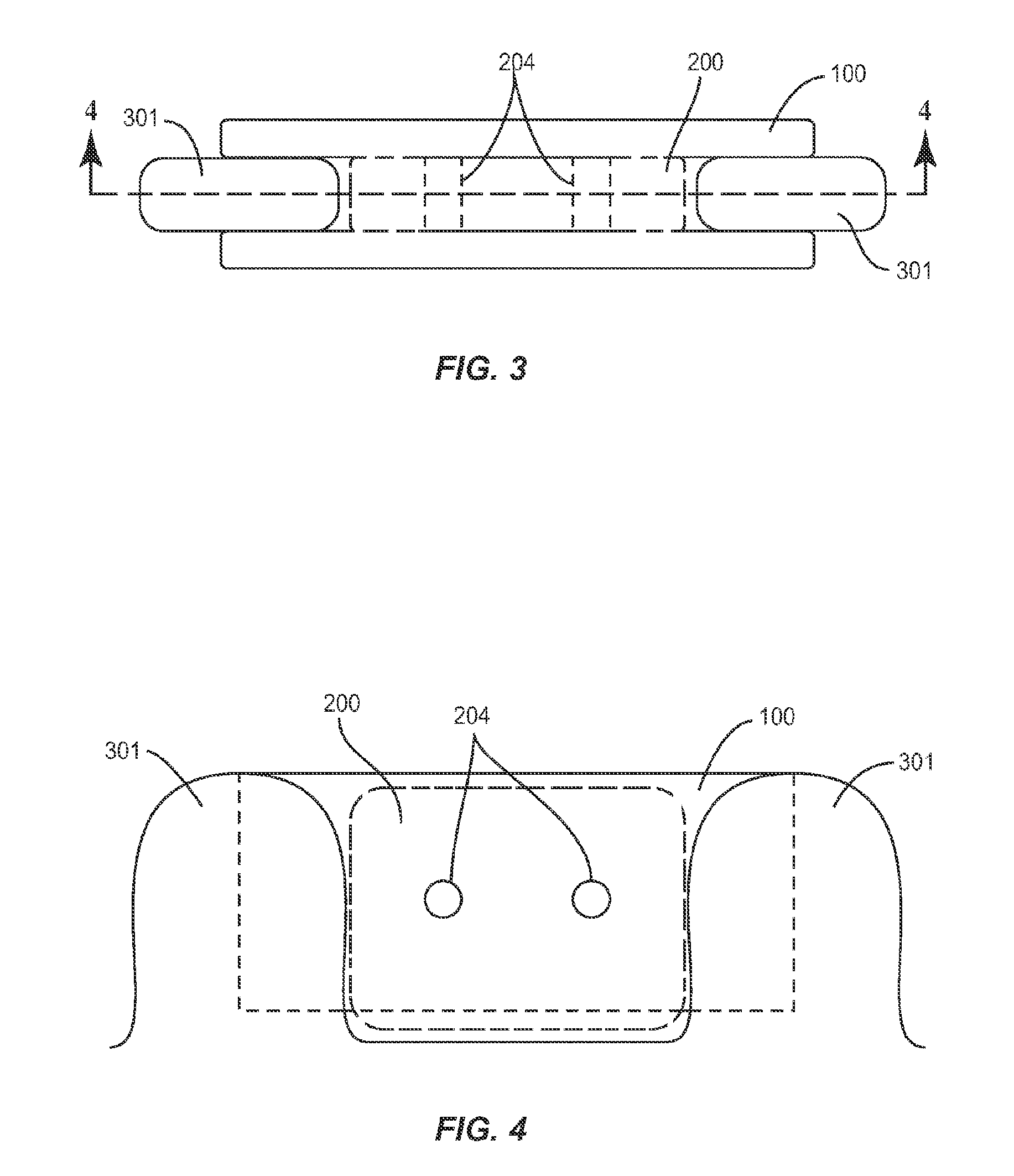
Spinal cage insert, filler piece and method of manufacturing
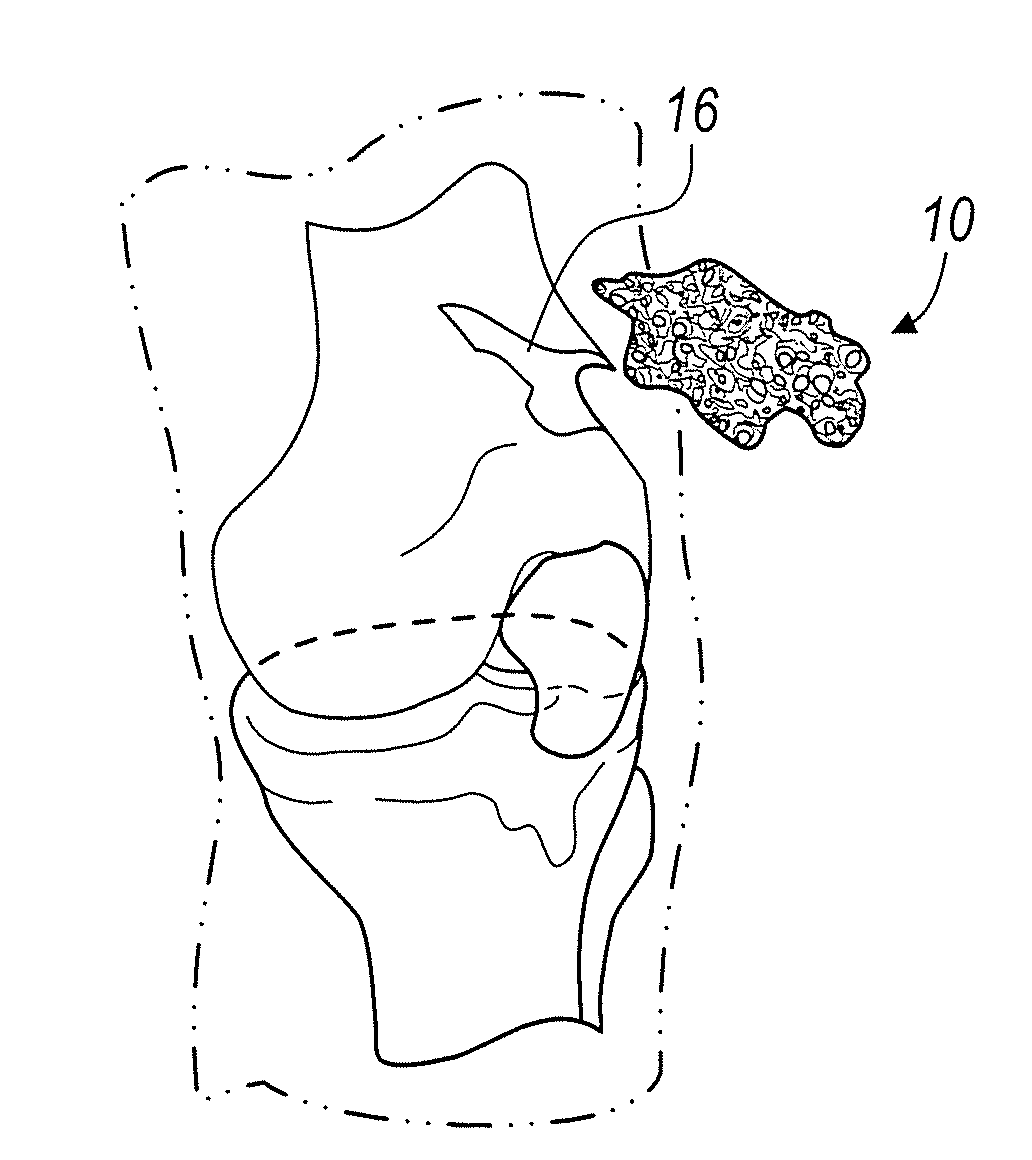
A spinal cage insert for a spinal cage is provided. The spinal cage insert has a shape suitable to be inserted into and fit closely in an interior of the spinal cage. The insert may comprise a member of the calcium phosphate family. The spinal cage insert may be made to a desired shape of porous ceramic, and it may include channels and / or surface features. Various shapes of filler pieces are also provided, wherein the filler pieces may be suitable to augment external regions of vertebrae which have been fused to each other so as to promote build-up of bone. The spinal cage insert and / or the filler pieces may be osteoconductive and may also contain osteoinductive substances or material. The articles may also contain cavities suitable for containing particles of demineralized bone matrix (DBM). Methods of use and methods of manufacturing the spinal cage insert and filler pieces are also provided.
Owner:THERICS
Crosslinked compositions comprising collagen and demineralized bone matrix, methods of making and methods of use
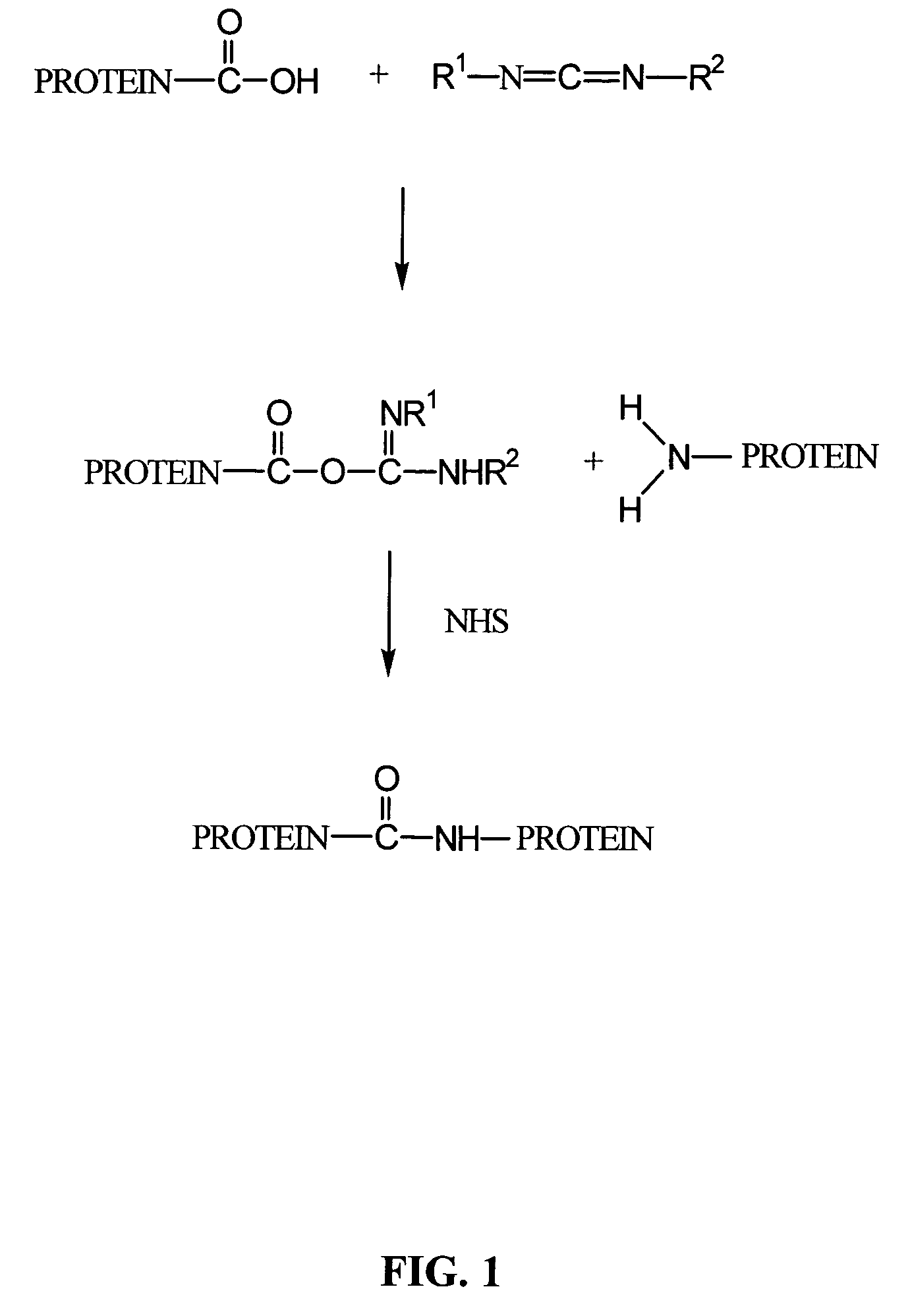
A composition comprising a collagen protein and demineralized bone matrix is described wherein the composition is chemically cross-linked with a carbodiimide such as N-(3-dimethylaminopropyl)-N-ethylcarbodiimide hydrochloride (EDC). The crosslinking reaction can be conducted in the presence of N-hydroxysuccinimide (NHS). The collagen can be in a porous matrix or scaffolding. The DBM can be in the form of particles dispersed in the collagen. A method of making the composition is also described wherein a collagen slurry is cast into the desired shape, freeze dried to form a porous scaffolding and infitrated with a solution comprising the cross-linking agent. The composition can be used as an implant for tissue (e.g., soft tissue or bone) engineering.
Owner:WARSAW ORTHOPEDIC INC
Osteoinductive demineralized cancellous bone
ActiveUS20090155378A1Increase osteoinductive activityHigh activityBone implantUnknown materialsBone implantDemineralized bone
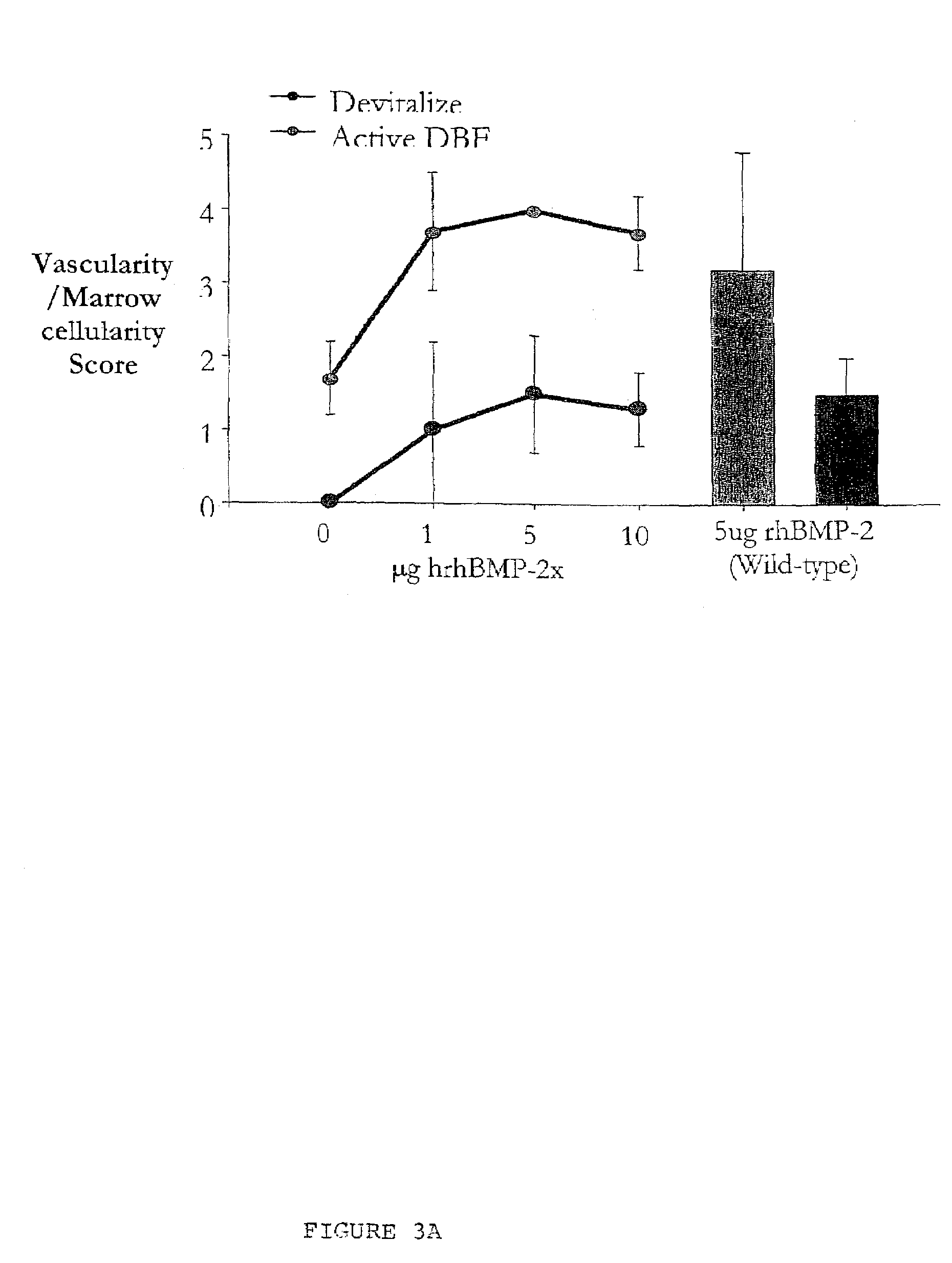
An osteoinductive demineralized bone matrix, corresponding osteoimplants, and methods for making the osteoinductive demineralized bone matrix are disclosed. The osteoinductive demineralized bone matrix may be prepared by providing demineralized bone and altering the collagenous structure of the bone. The osteoinductive demineralized bone matrix may also be prepared by providing demineralized bone and compacting the bone, for example via mechanical compaction, grinding into a particulate, or treatment with a chemical. Additives such as growth factors or bioactive agents may be added to the osteoinductive demineralized bone matrix. The osteoinductive demineralized bone matrix may form an osteogenic osteoimplant. The osteoimplant, when implanted in a mammalian body, may induce at the locus of the implant the full developmental cascade of endochondral bone formation including vascularization, mineralization, and bone marrow differentiation. The osteoinductive demineralized bone matrix may also be used as a delivery device to administer bioactive agents.
Owner:WARSAW ORTHOPEDIC INC
Osteoinductive bone material
ActiveUS20050084542A1Sufficient amountMaximize formabilityBiocideInorganic phosphorous active ingredientsNatural boneBone implant
Osteogenic bone implant compositions that approximate the chemical composition of natural bone are provided. The organic component of these implant compositions is osteoinductive despite the presence of the inorganic component and, further, is present in an amount sufficient to maximize the regenerative capabilities of the implant without compromising its formability and mechanical strength. The composition may be an osteoinductive powder, including demineralized bone matrix (DBM) particles, a calcium phosphate powder, and, optionally, a biocompatible cohesiveness agent. The powder may be combined with a physiologically-acceptable fluid to produce a formable, osteoinductive paste that self-hardens to form a poorly crystalline apatitic (PCA) calcium phosphate having significant compressive strength. The bone implant materials retain their cohesiveness when introduced at an implant site and are remodeled into bone in vivo. Methods for using these implant materials to repair damaged bone and a method of assaying the content of DBM particles, by weight, in a bone implant material are also provided.
Owner:ETEX
Implantable biostructure comprising an osteoconductive member and an osteoinductive material
The present invention is directed to a biostructure comprising an osteoconductive member and an osteoinductive material. The osteoinductive material may be located within a cavity in the osteoconductive material. In one aspect of the invention the osteoinductive material is demineralized bone matrix and the osteoconductive member comprises tricalcium phosphate.
Owner:THERICS
Resorbable bone graft materials
Ceramic materials operable to repair a defect in bone of a human or animal subject comprising a porous ceramic scaffold having a bioresorbable coating, and a carrier comprising denatured demineralized bone. The ceramic may contain a material selected from the group consisting of hydroxyapatite, tricalcium phosphate, calcium phosphates, calcium carbonates, calcium sulfates, and combinations thereof. The compositions may also contain a bone material selected from the group consisting of: bone powder, bone chips, bone shavings, and combinations thereof. The bioresorbable coating may be, for example, demineralized bone matrix, gelatin, collagen, hyaluronic acid, chitosan, polyglycolic acid, polylactic acid, polypropylenefumarate, polyethylene glycol, or mixtures thereof.
Owner:BIOMET MFG CORP
Cohesive demineralized bone compositions
ActiveUS7582309B2Keep shapeEnhance bone ingrowth and remodelingPowder deliverySurgical adhesivesFiberDemineralized bone matrix
Demineralized bone matrix fibers and a demineralized bone matrix composition are provided. The demineralized bone matrix fibers have an average fiber length in the range from about 250 μm to about 2 mm and an aspect ratio of greater than about 4. The demineralized bone matrix composition includes demineralized bone matrix fibers and a biocompatible liquid in an amount to produce a coherent, formable mass. The formable mass retains its cohesiveness when immersed in a liquid. Methods for making the demineralized bone matrix fibers and composition are also provided.
Owner:ETEX
Stabilized bone graft
ActiveUS20070178158A1Good osteoinductivityImprove stabilityOrganic active ingredientsBiocideProteinase activityGlycerol
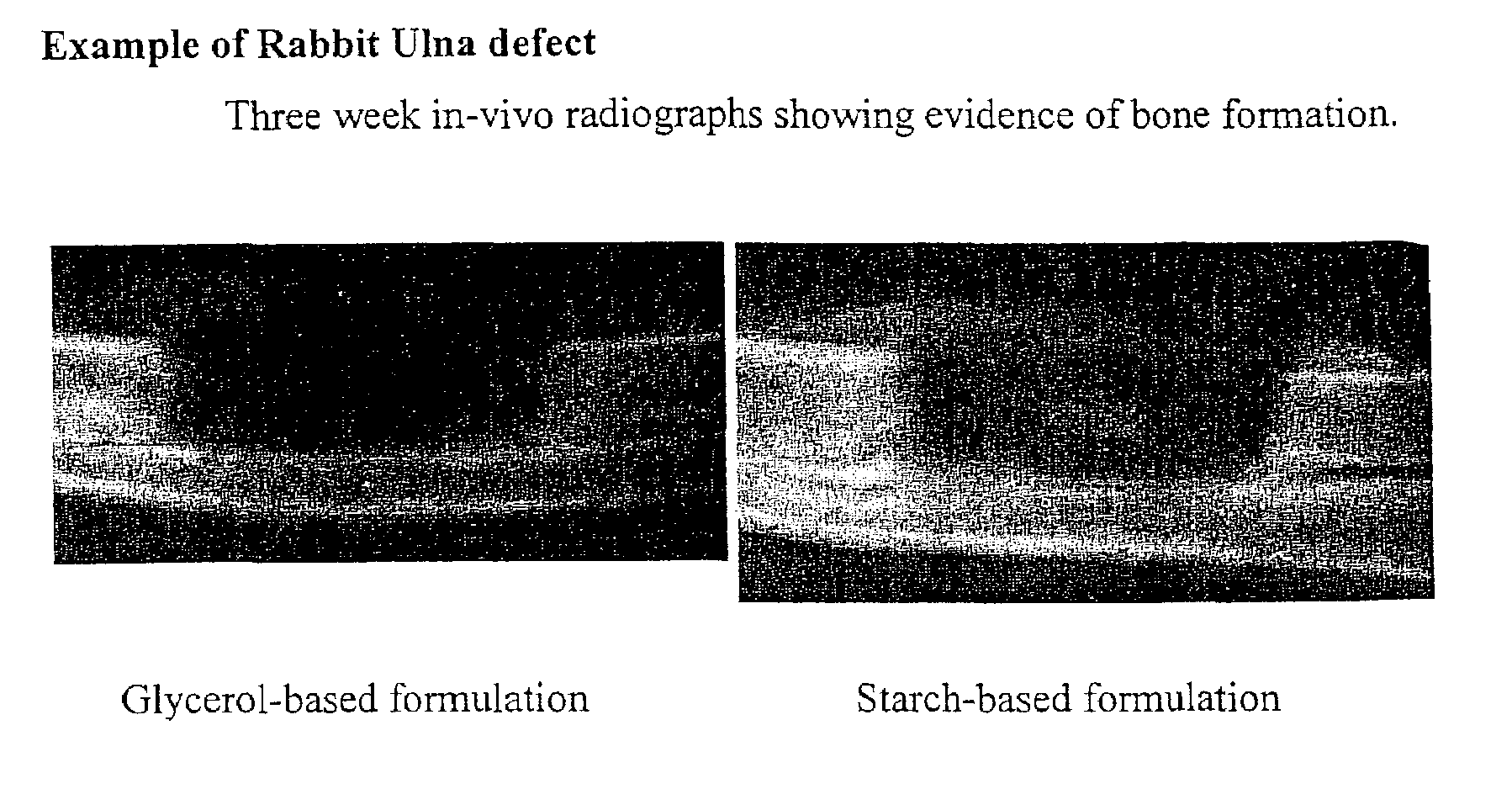
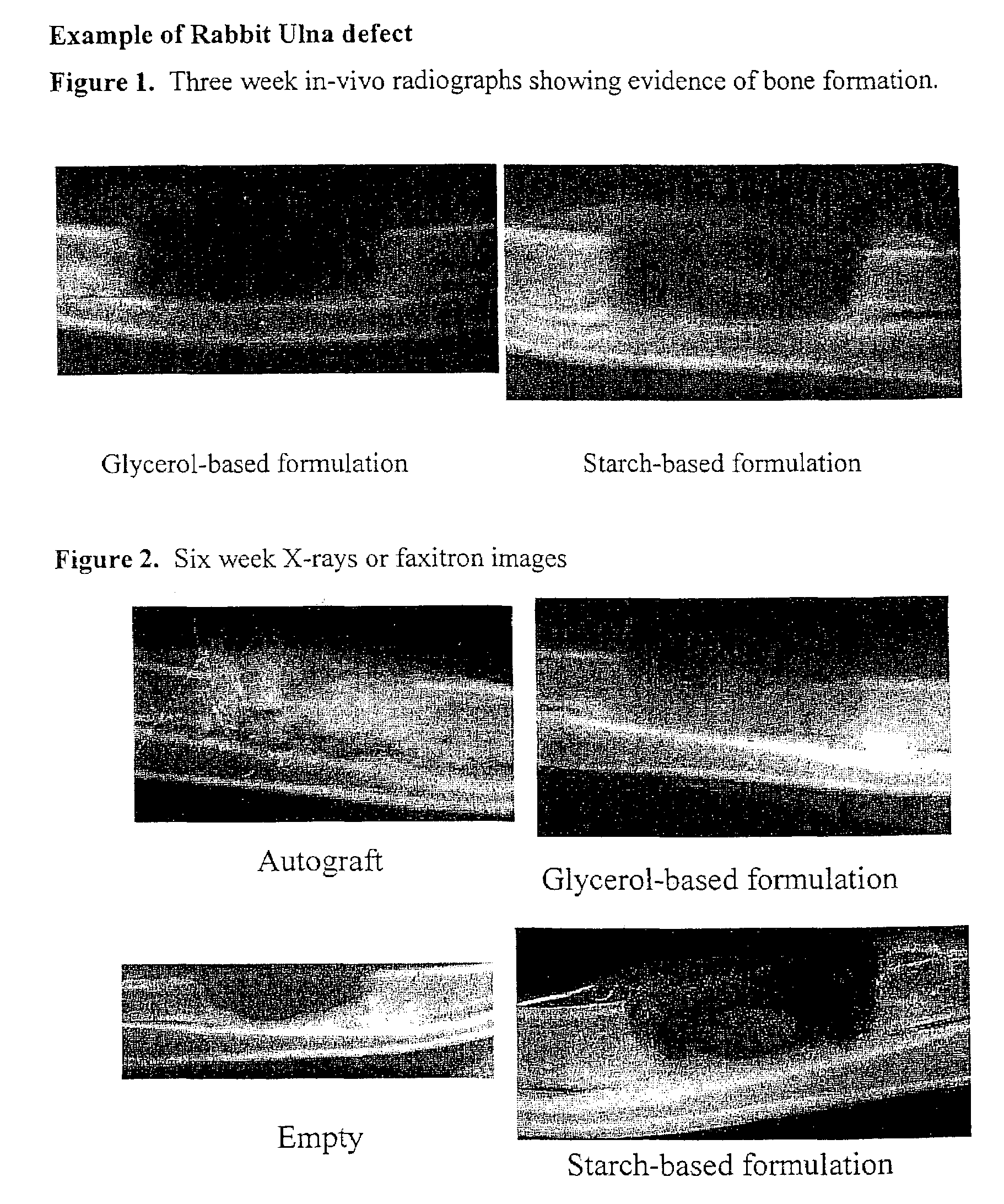
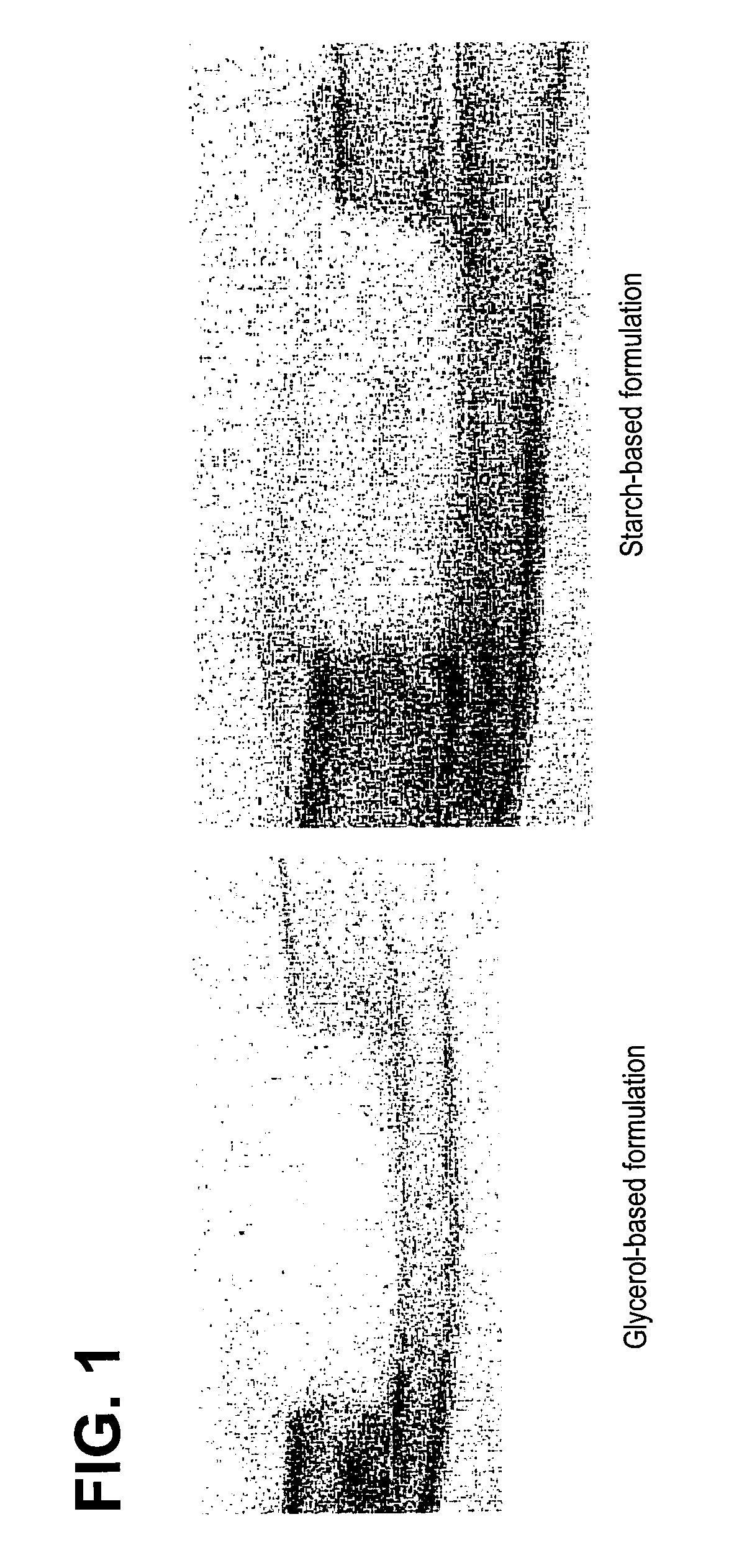
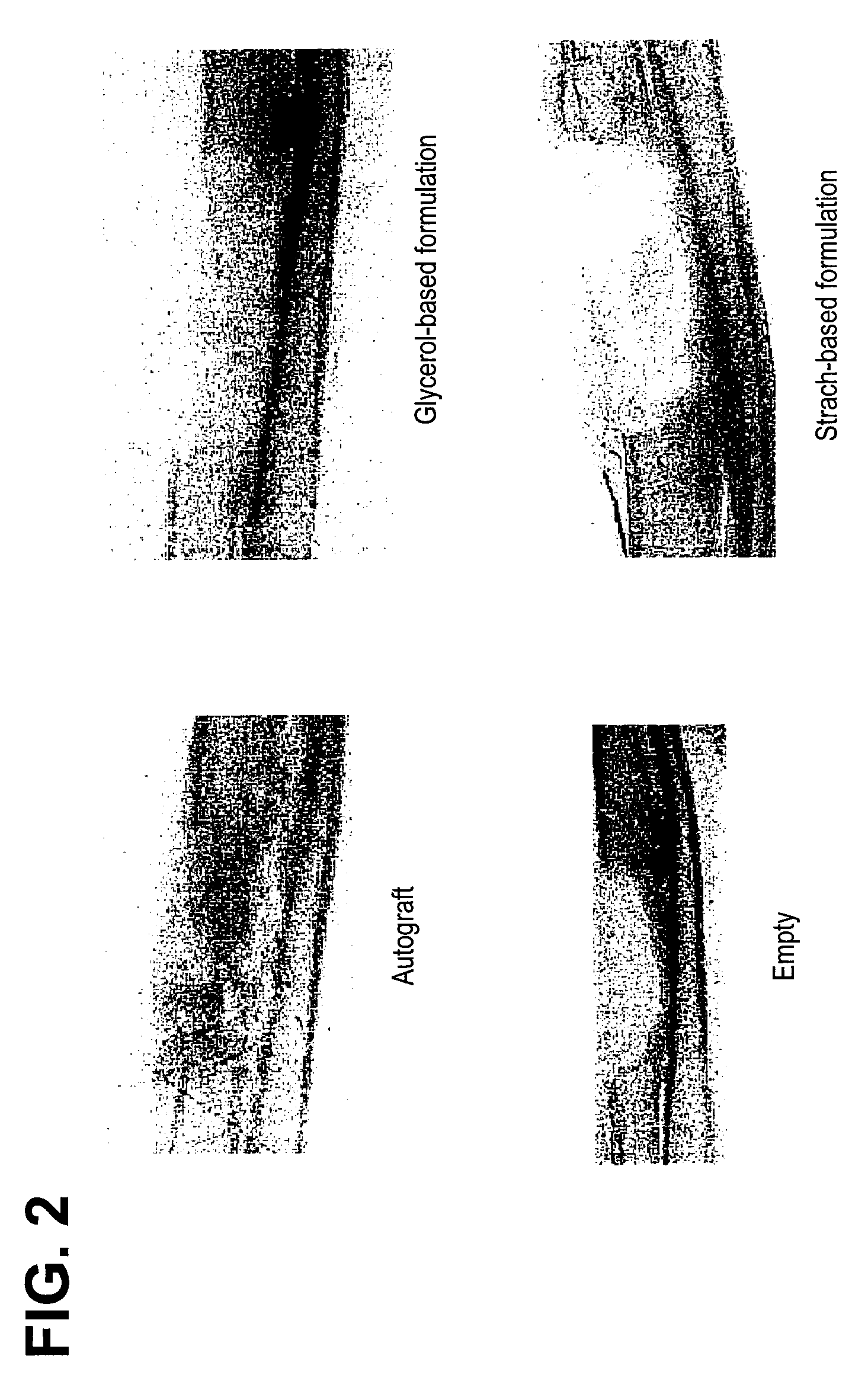
A demineralized bone matrix (DBM) or other matrix composition is provided that has been stabilized by lowering the pH of the composition, reducing the water content, adding water substitutes, and / or increasing the amount of deuterated water present in the composition in order to reduce the activity of endogenous degrading enzymes such as proteases. A hydrated form of a stabilized DBM composition may be stable up to a year at room temperature at acidic pH. The acidified DBM compositions may be further stabilized by the addition of a stabilizing agent such as deuterated water, water substitutes, polymers, protease inhibitors, glycerol, hydrogels, etc. The invention also provides methods of preparing, testing, and using the inventive stabilized osteodinductive matrix compositions.
Owner:WARSAW ORTHOPEDIC INC
Bone graft material incorporating demineralized bone matrix and lipids
InactiveUS6565884B2Good osteoinductivityEasy to operateBiocidePowder deliveryHydrophilic polymersVitamin C
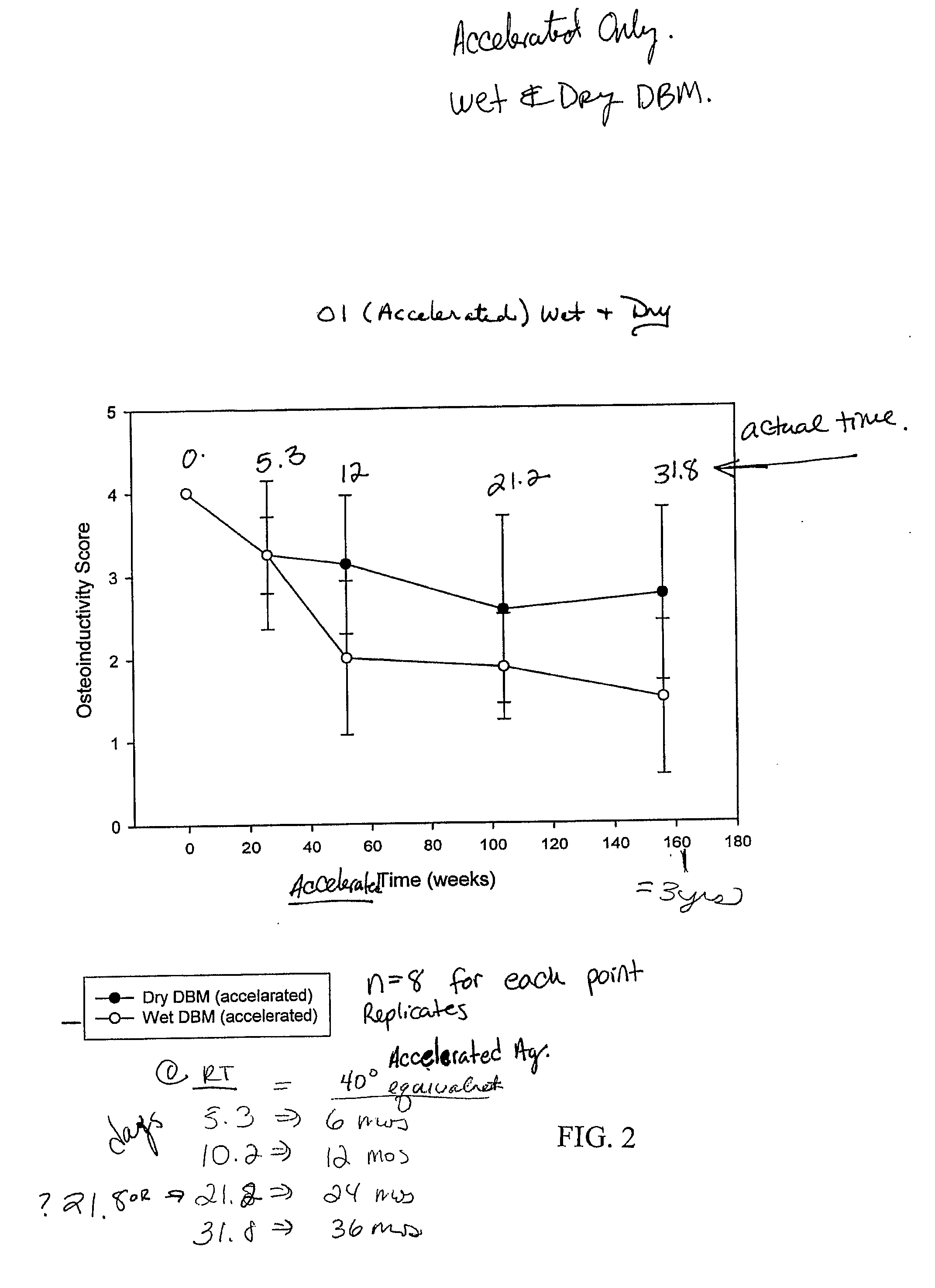
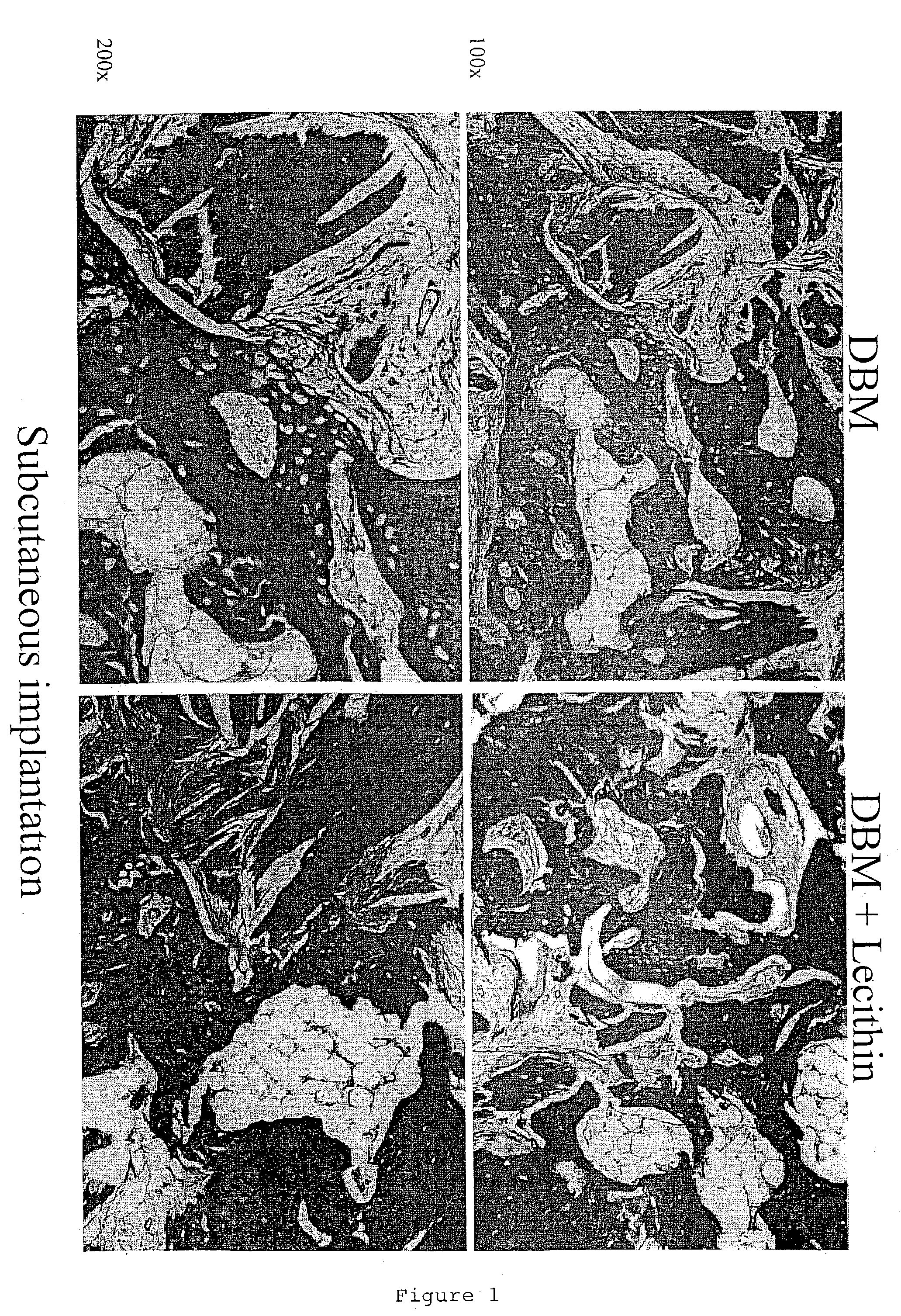
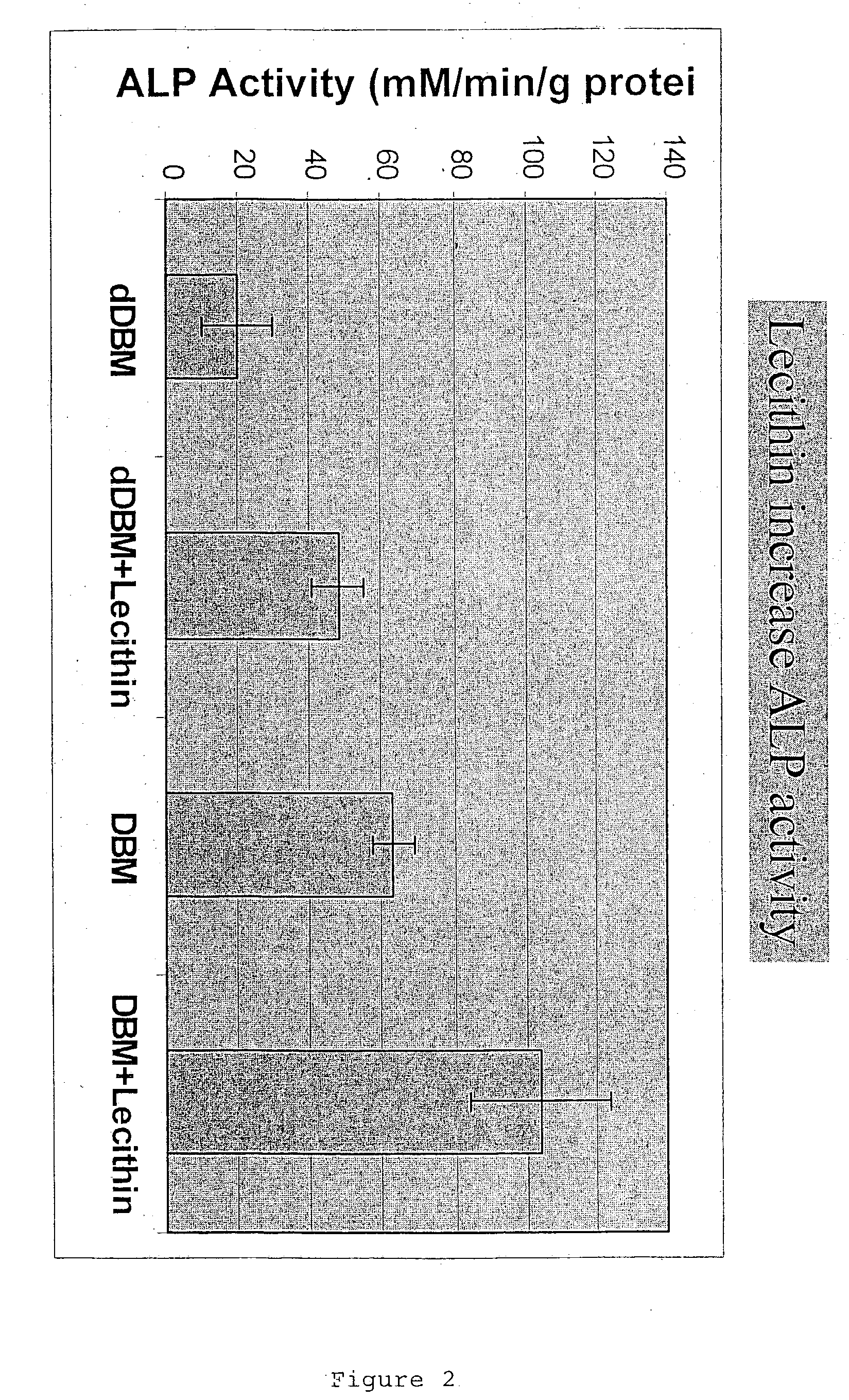
A demineralized bone putty composition comprises: (1) demineralized bone matrix (DBM); and (2) a lipid fraction selected from the group consisting of lecithin and a mixture of lecithin and triglycerides containing unsaturated fatty acids. The putty composition is moldable, biocompatible, slowly resorbable, and soluble in tissue fluids, and non-extrudable. The composition delivers a biologically active product to animals and humans that will enhance bone formation at sites where bone is lost, deficient, or present in suboptimal amounts. The composition can further comprise calcium, an antioxidant such as Vitamin E or Vitamin C, or a hydrophilic polymer such as methylcellulose or hydroxypropyl methylcellulose.
Owner:BIOMET MFG CORP
Demineralized bone matrix compositions and methods
Bone matrix compositions and, more specifically, demineralized bone matrix (DBM) having increased osteoinductive capacity and methods for its production are provided. Specifically, DBM derived from cortical bone from the periosteal layer of bone are provided. Compositions comprising a disproportionate amount of DBM prepared from bone derived from the periosteal and / or middle layer of bone are provided. Preparations of and methods of use of periosteal DBM compositions are disclosed.
Owner:WARSAW ORTHOPEDIC INC
Bone void filling tube and shear mechanism
Owner:ARTHREX
Method of preparing bone material having enhanced osteoinductivity
ActiveUS20140205674A1Good osteoinductivityIncrease surface areaPeptide/protein ingredientsMammal material medical ingredientsFiberCritical point drying
Methods for increasing osteoinductivity and / or surface area of bone material are provided. The methods include providing bone material and dehydrating the bone material with a solvent at its critical point. A useful solvent for critical point dehydrating is carbon dioxide. Critical point dehydration resulting in increased osteoinductivity and / or surface area can be applied to many types of bone material including bone particles, bone chips, bone fibers, bone matrices, both demineralized and non-demineralized. An implantable composition having an enhanced osteoinductivity and / or osteoconductivity is also provided. The implantable composition contains demineralized bone matrix dried at critical point of carbon dioxide. Critical point dried fibers of a demineralized bone matrix have a BET value from about 40 m2 / gm to about 100 m2 / gm, a value that is 100 times higher than corresponding vacuum dried or lyophilized DBM fibers.
Owner:WARSAW ORTHOPEDIC INC
Transplantable particulate bone composition having high osteoinductive capacity and methods for making and using same
ActiveUS7335381B2High activityIncrease capacitySurgical adhesivesBone implantEndochondral ossificationParticulates
A particulate bone of defined sizes which has a unique property of direct bone osteoinduction without going through endochondral ossification stage is disclosed. The bone is not subjected to chemical extraction or decalcification. This allows for the retention of all physiologically active components of native bone. The invention hinges on the newly discovered ability of bone particles of defined sizes to exert osteoinduction by a pathway superior to and distinct from demineralized bone matrix and similar preparations.
Owner:VIVEX BIOLOGICS GRP INC
Demineralized bone matrix compositions and methods
ActiveUS20090226523A1Good osteoinductivityPowder deliverySkeletal disorderBone CortexDemineralized bone matrix
Bone matrix compositions and, more specifically, demineralized bone matrix (DBM) having increased osteoinductive capacity and methods for its production are provided. Specifically, DBM derived from cortical bone from the periosteal layer of bone are provided. Compositions comprising a disproportionate amount of DBM prepared from bone derived from the periosteal and / or middle layer of bone are provided. Preparations of and methods of use of periosteal DBM compositions are disclosed.
Owner:WARSAW ORTHOPEDIC INC
Tissue integration implant
The present invention relates to a tissue defect repair implant including a porous material, wherein the material is expandable or compressible. The implant may be a scaffold, and may also be formed of a material such as, for example, collagen or demineralized bone matrix. Another aspect of the invention may be a method for the repair of a tissue defect in a patient in need thereof, including implanting an implant between two adjacent tissues, wherein the implant may include a porous material which is expandable or compressible. The two tissues may be soft tissue and hard tissue, and alternatively, the two tissues may be soft tissue, and may further be the same or different soft tissues.
Owner:HOWMEDICA OSTEONICS CORP
Bone Graft
ActiveUS20080145392A1Good curative effectSimple compositionAdditive manufacturing apparatusBone implantOSTEOINDUCTIVE FACTORIn vivo
An improved demineralized bone matrix (DBM) or other matrix composition is provided that has been mixed with a stabilizing agent that acts as (1) a diffusion barrier, (2) a enzyme inhibitor, (3) a competitive substrate, or (4) a masking moiety. A diffusion barrier acts as a barrier so as to protect the osteoinductive factors found in DBM from being degraded by proteolytic and glycolytic enzymes at the implantation site. Stabilizing agents may be any biodegradable material such as starches, modified starches, cellulose, dextran, polymers, proteins, and collagen. As the stabilizing agents degrades or dissolves in vivo, the osteoinductive factors such as TGF-.beta., BMP, and IGF are activated or exposed, and the activated factors work to recruit cells from the preivascular space to the site of injury and to cause differentiation into bone-forming cells. The invention also provides methods of preparing, testing, and using the inventive improved osteodinductive matrix compositions
Owner:WARSAW ORTHOPEDIC INC
Interspinous bone implant device
ActiveUS8771368B2Enhanced osteogenic propertiesImproved and facilitatedInternal osteosythesisBone implantMedicineBone implant
A bone implant system is provided including a bone graft body having at least a demineralized surface for implantation at a bone site, the bone graft body including at least one attachment member. At least one bone delivery carrier including a covering is provided, the carrier configured to be attachable to the bone graft body and including at least one compartment. An affixing member may be provided for attaching the at least one bone delivery carrier to the at least one attachment member of the bone graft body. A demineralized bone matrix material is disposed within the at least one compartment, wherein the covering retains the demineralized bone matrix material for placement at the bone site and facilitates transfer of cells into and out of the covering.
Owner:WARSAW ORTHOPEDIC INC
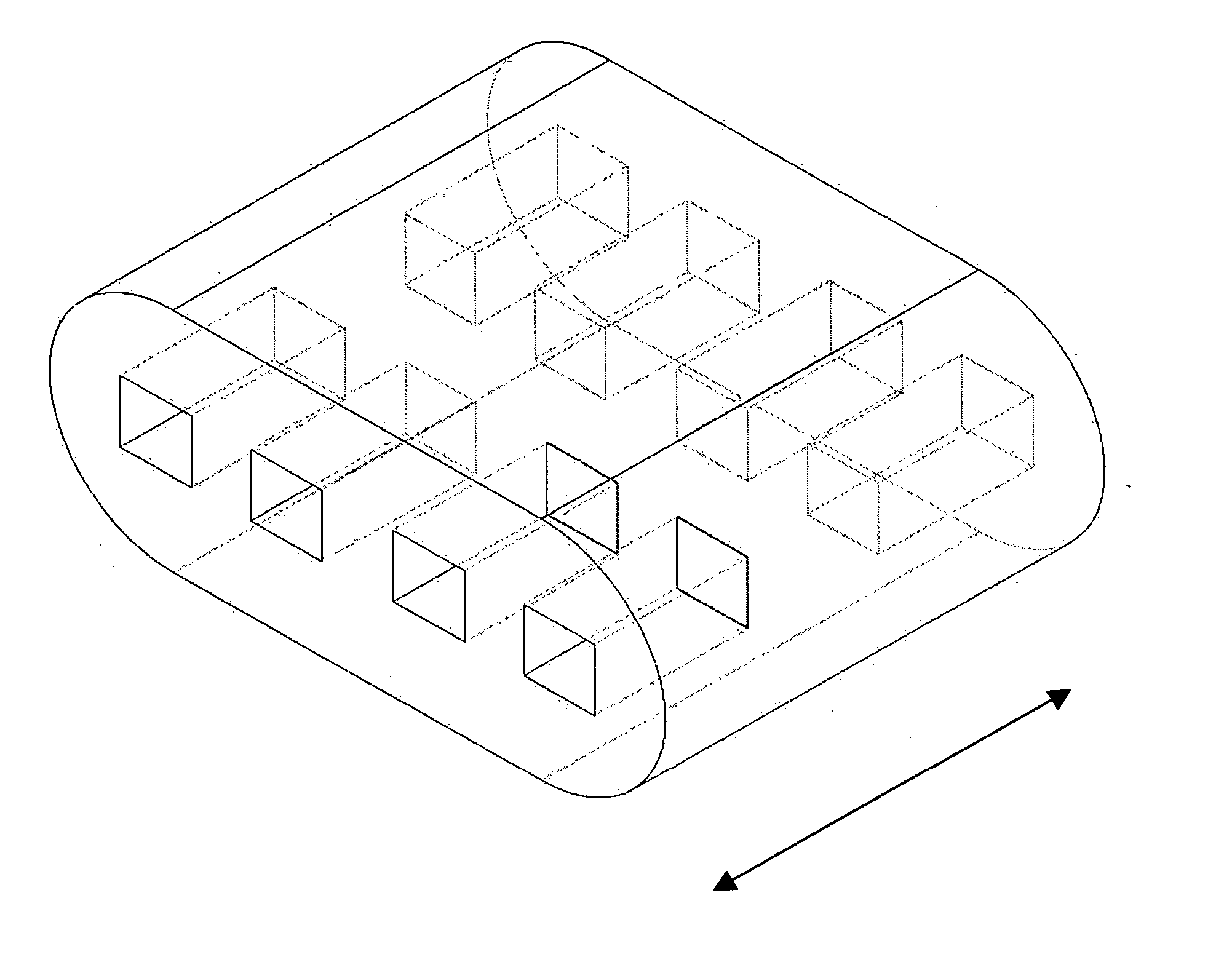
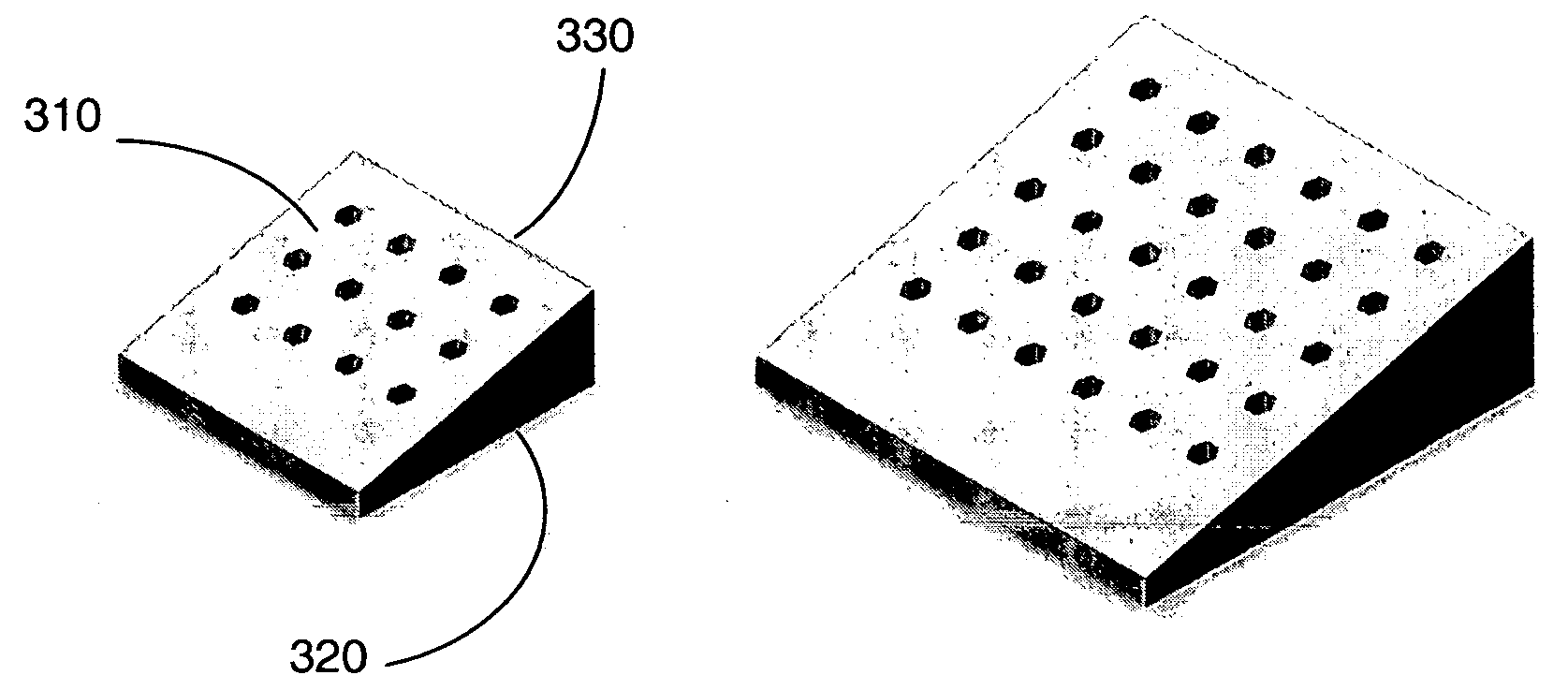
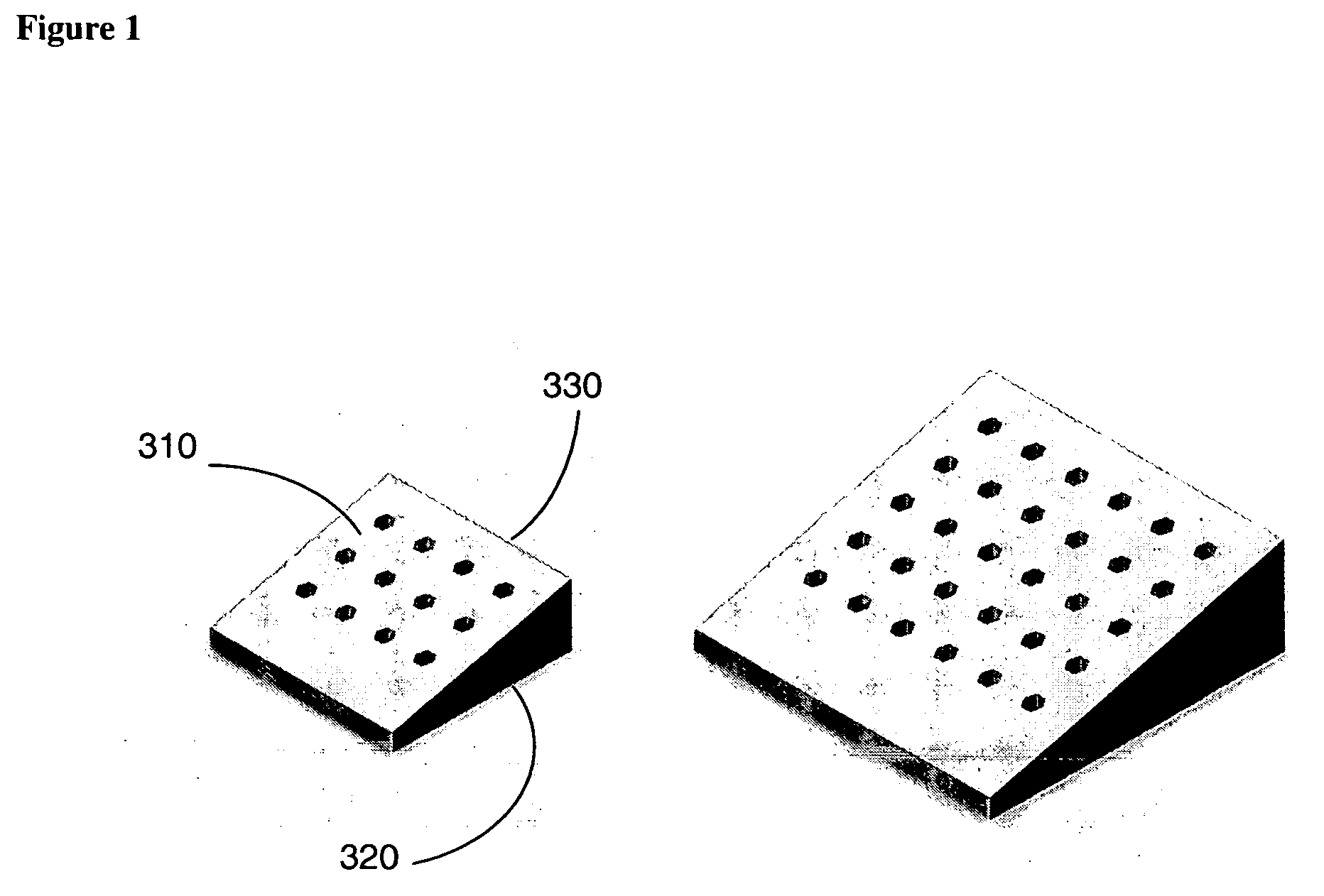
Shaped filler for implantation into a bone void and methods of manufacture and use thereof
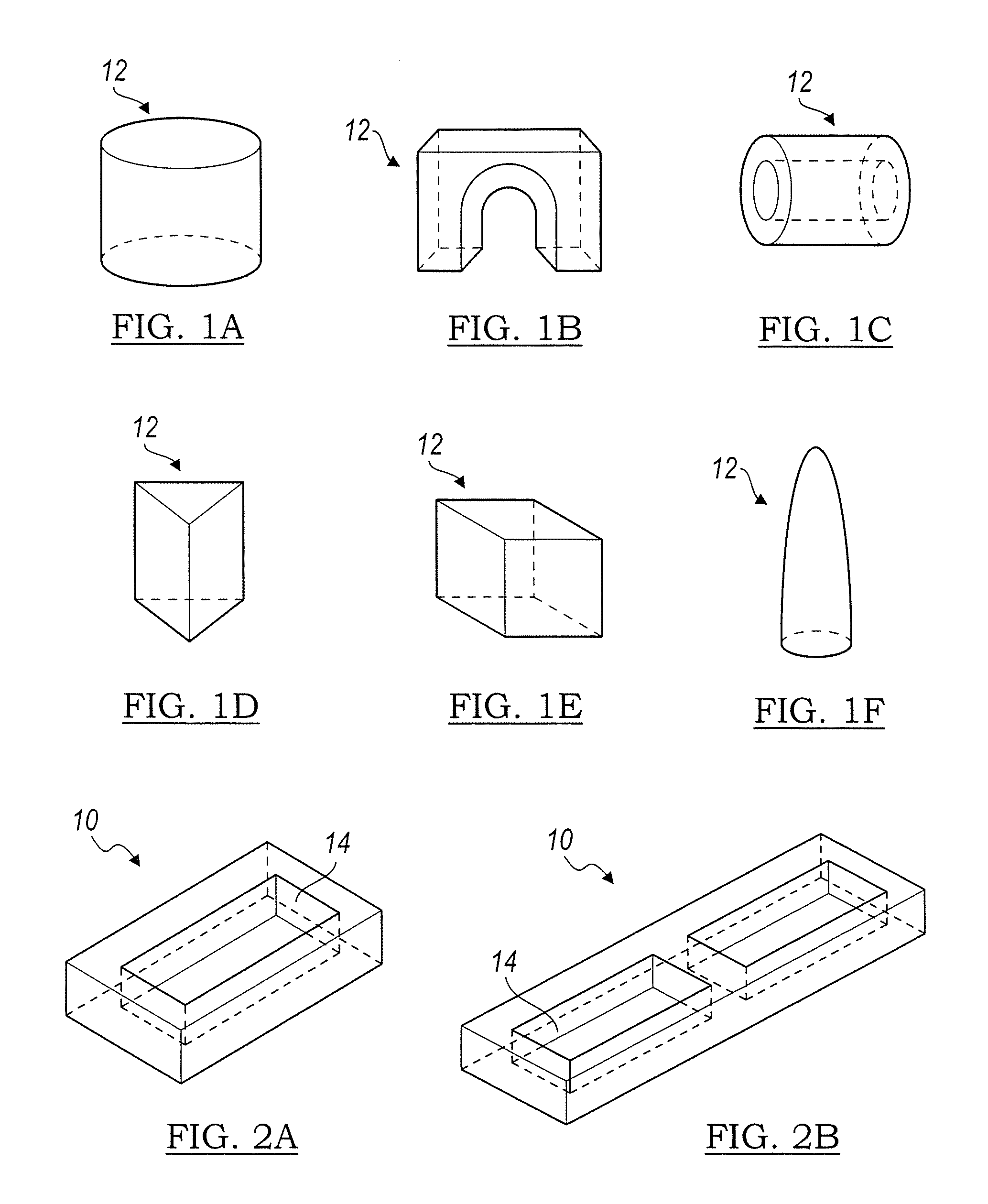
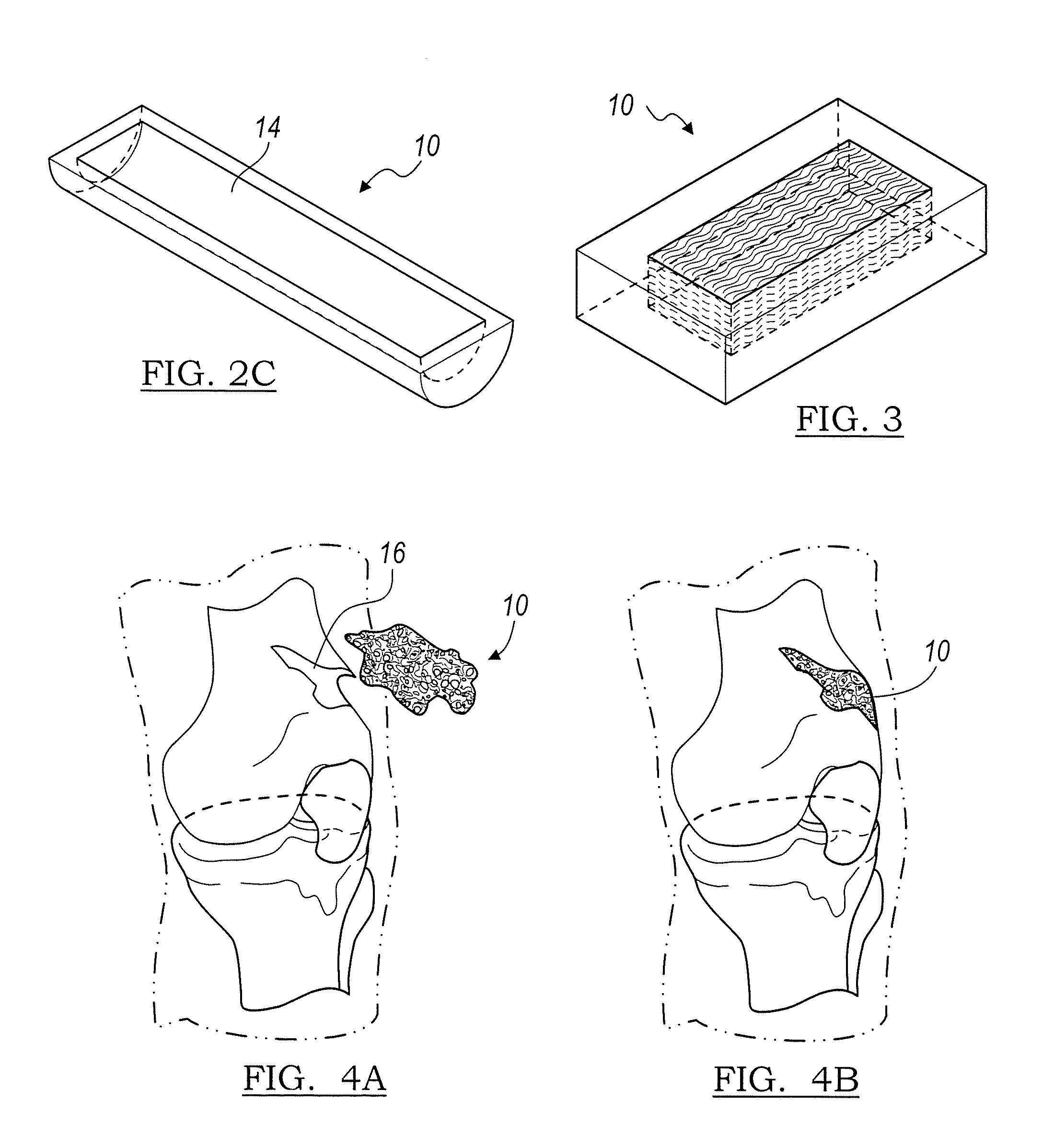
The invention is directed to shaped bone void filler pieces having defined porosity. In embodiments of the invention, the shaped bone void filler pieces are presented substantially as wedges, wafers, and axisymmetric bone void filler pieces. The bone void filler pieces further comprise surface and internal features such as recesses, channels, and / or voids. The bone void filler pieces optionally comprise demineralized bone matrix. The invention further is directed to methods of making and methods of using the bone void filler pieces. In another embodiment of the invention, the bone void filler pieces are produced using three dimensional printing methods. In yet another embodiment of the invention, the bone void filler pieces are manufactured with selected porogens integrated therein, which optionally are decomposed following production through a heat-mediated decomposition process, resulting in voids in the bone void filler spaces previously occupied by the porogen(s).
Owner:THERICS
Method of manufacture, installation, and system for a sinus lift bone graft
A bone graft that is made at least partially of synthetic material or demineralized bone matrix and is of a suitable shape to fill a lower portion of a patient's maxillary sinus. The graft may be manufactured to patient-unique dimensions that may be determined radiographically prior to surgery and prior to manufacturing of the bone graft. Other aspects of the invention are a method of manufacture of the bone graft, and methods of installing the bone graft. The installation may make use of patient-unique templates to guide certain cutting operations. Another aspect of the invention is a kit comprising the bone graft, tools for its installation, templates and possibly other surgical items.
Owner:THERICS
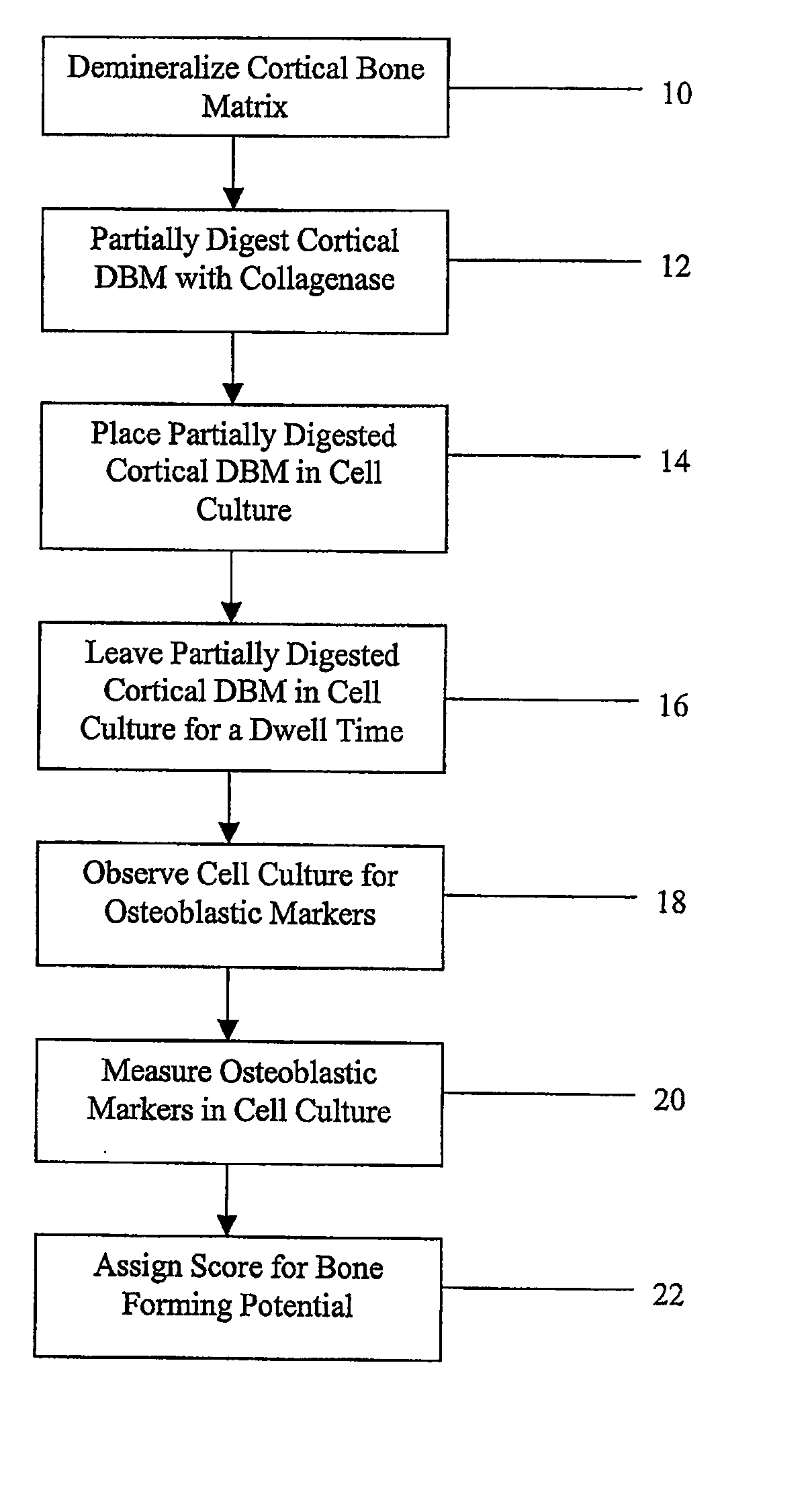
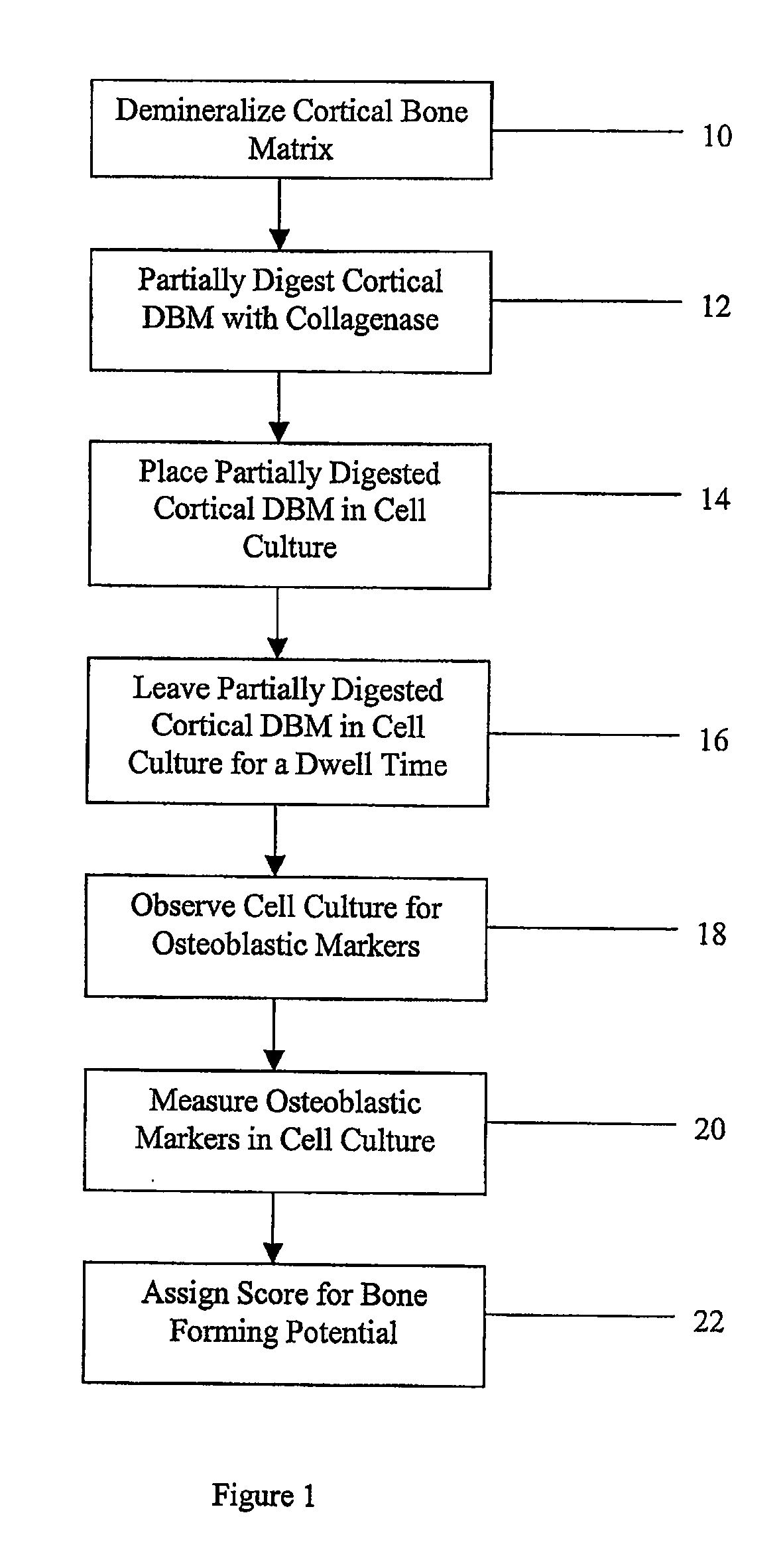
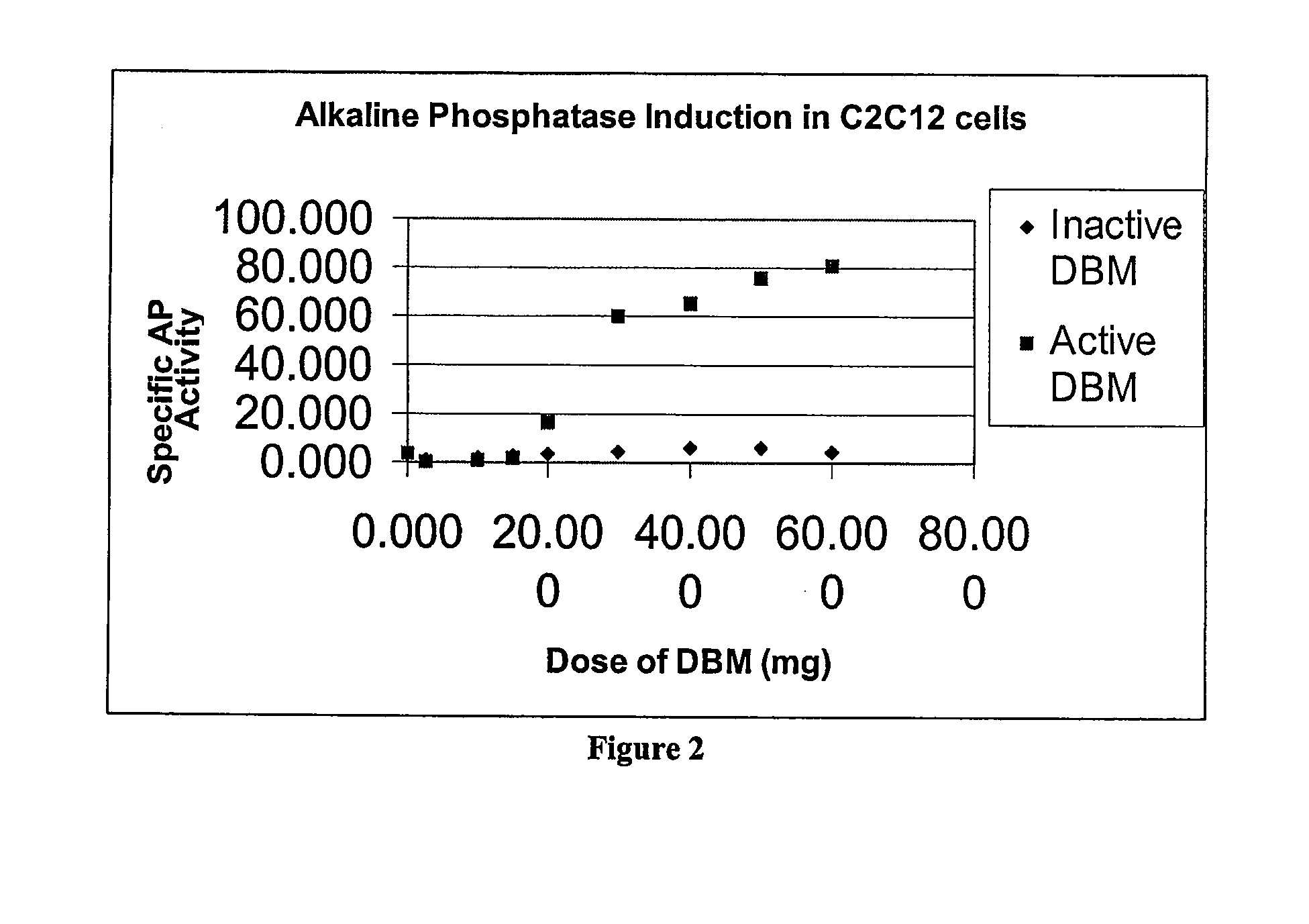
Method for In Vitro Assay of Demineralized Bone Matrix
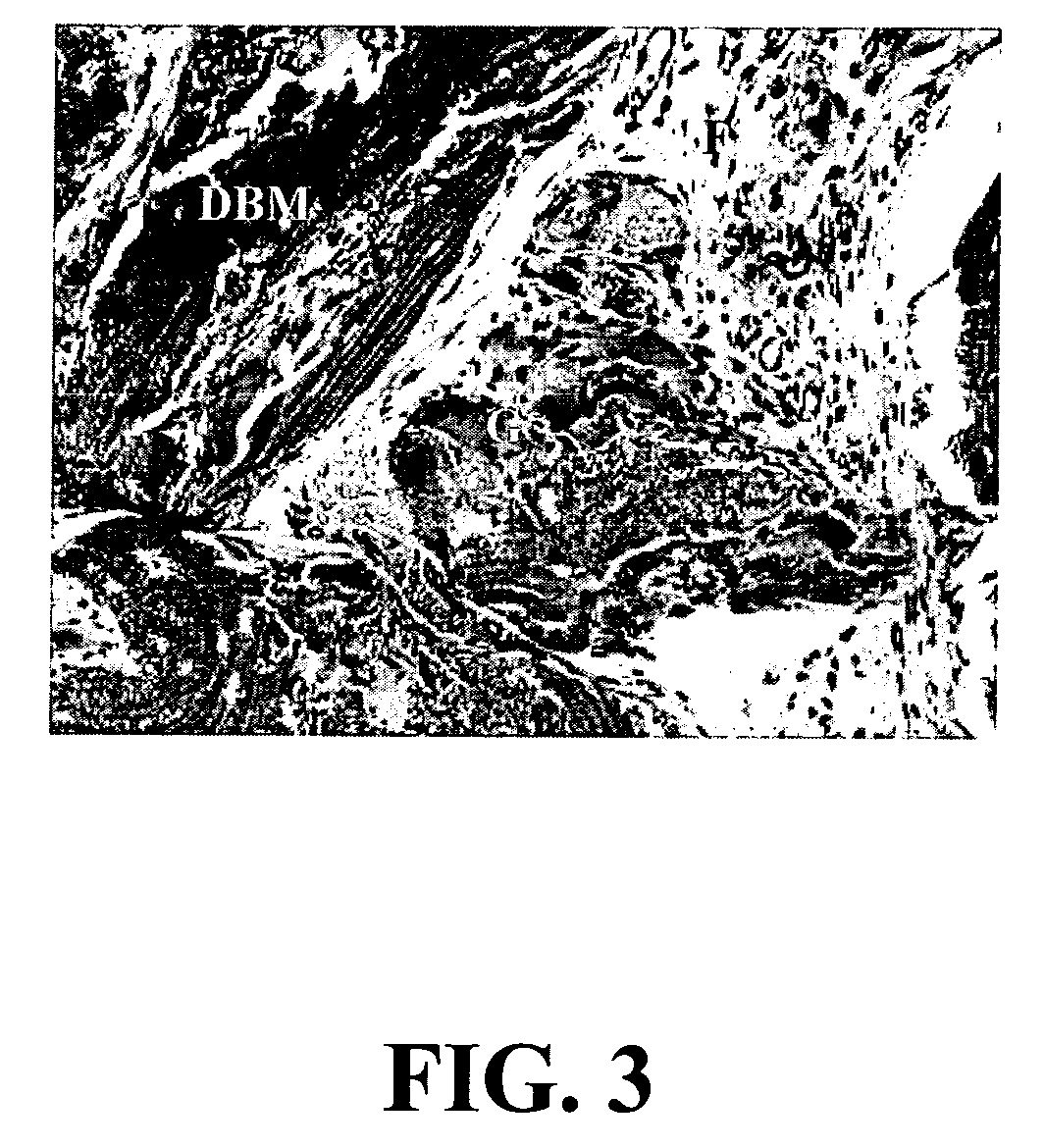
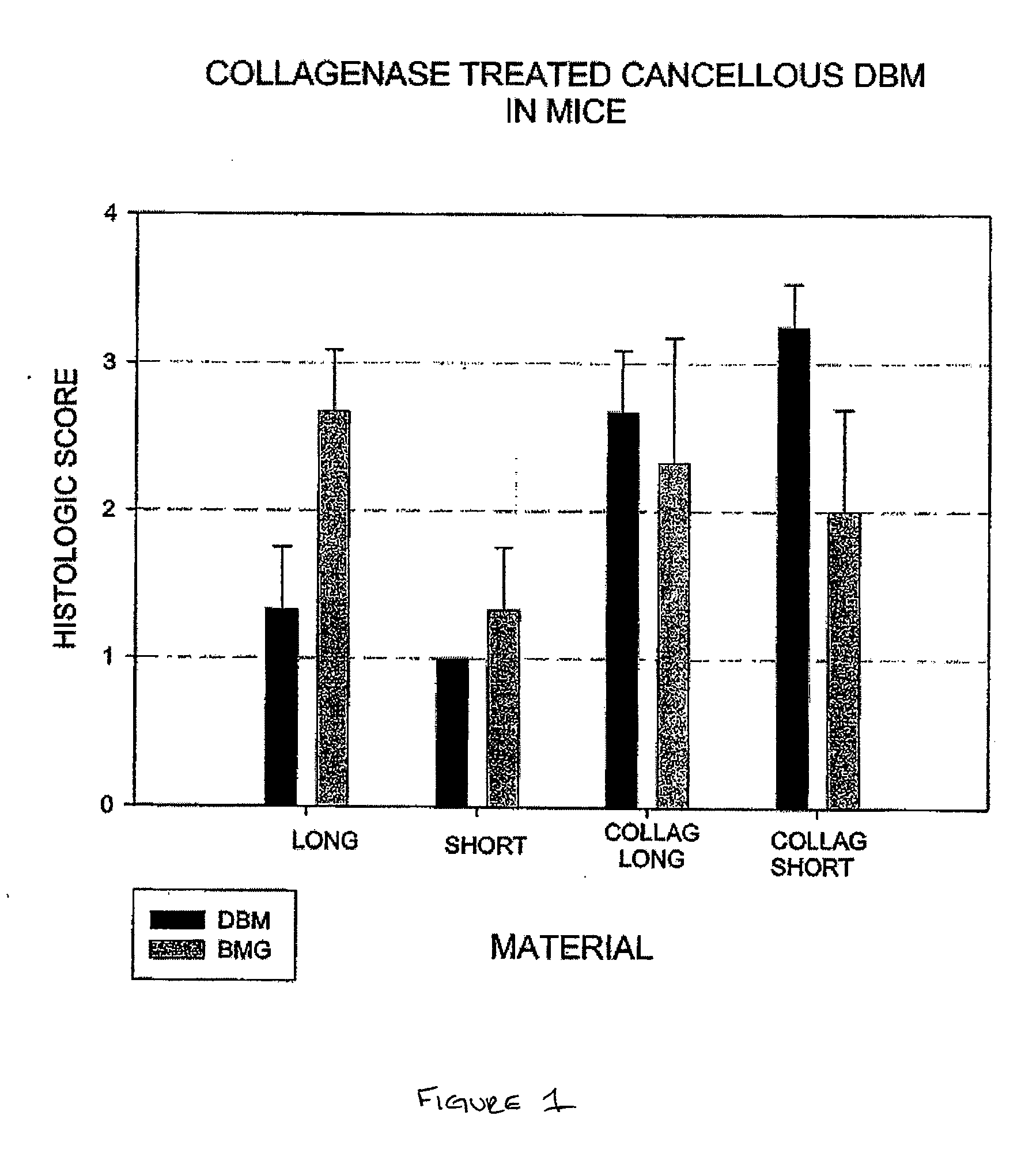
A sensitive, quantitative method to assess bone forming potential of demineralized bone matrix (DBM) is provided. Cortical DBM is treated with collagenase. The treated cortical DBM is placed in a cell culture for a dwell time. After the dwell time, the cell culture is observed for osteoblastic markers. The cortical bone so treated exhibits the same bone forming potential in vivo as untreated cortical bone.
Owner:WARSAW ORTHOPEDIC INC
Hemostatic bone graft
ActiveUS20080063671A1Reduce stepsInduce and osteoinductive activitySurgical adhesivesPharmaceutical delivery mechanismPolyethylene glycolTonicity
The present invention provides a hemostatic bone graft product and method. Hemostatic bone grafts may include demineralized bone matrix in combination with additives. In one embodiment, the graft comprises demineralized bone and polyethylene glycol. Methods for producing the hemostatic bone graft may include mixing demineralized bone with additives to facilitate protein precipitation, surface tension reduction in blood, and / or a cytolytic effect on cells at a bleeding site.
Owner:WARSAW ORTHOPEDIC INC
Osteoinductive bone graft injectable cement
ActiveUS20120100225A1Sufficient load bearingFoster de novo bone growthSurgical adhesivesPeptide/protein ingredientsFiberMedicine
Osteoconductive bone graft materials are provided. These compositions contain injectable cements and demineralized bone matrix fibers. The combination of these materials enables the filling of a bone void while balancing strength and resorption.
Owner:WARSAW ORTHOPEDIC INC
Novel biological implant compositions, implants and methods
The present application is directed to the field of implants comprising processed hard or soft biological tissues for use in implantation in humans. The molded biological tissue implants of the present application are preferably made from allograft soft tissue sources and demineralized bone matrix. The present application provides biological implants exhibiting advantageous properties of absorption, expansion, resiliency and shape retention. The properties of the biological implants produced by the methods of the present application are leveraged in the creation and development of novel biological implant constructs and of novel methods of treatment and surgical technique.
Owner:RTI BIOLOGICS INC
Bone filler material
ActiveUS20100255115A1Facilitate re-hydrationPowder deliveryPeptide/protein ingredientsGellan gumSphingomonas elodea
Described are bone generation matrixes having an admixture of demineralized bone matrix (DBM) particles and / or bone chips in combination with at least one binding agent selected from the group consisting of alginate, lectin, pectin, gellan gum, starch, collagen and combinations thereof in an aqueous solvent, wherein the DBM particles and / or bone chips are present in an amount of at least 65% by dry weight and the ratio of the aqueous solvent to the dry weight of the DBM particles / bone chips and at least one binding agent is between about 1:1 to about 1:6.
Owner:WARSAW ORTHOPEDIC INC
Method of manufacture, installation, and system for an alveolar ridge augmentation graft
A bone graft that is made at least partially of synthetic material or demineralized bone matrix and is manufactured in suitable shape and / or dimensions to augment an alveolar ridge. The bone graft may be such as to augment both a portion of the crest of the alveolar ridge and a portion of at least one side of the alveolar ridge. The graft may include at least one hole for the intended position of an implant base, and / or at least one hole for attachment hardware. The graft may be manufactured to standard dimensions or it may be manufactured to patient-unique dimensions which may be determined radiographically prior to surgery and prior to manufacturing of the bone graft. The bone graft may be able to be carved for dimensional adjustment during surgery. The bone graft may have composition and / or local geometry which varies from one place to another, and may have a particular composition and / or local geometry at places intended to adjoin natural bone.
Owner:THERICS
Bone graft material incorporating demineralized bone matrix and lipids
InactiveUS20040091459A1Good osteoinductivityEasy to operateBiocidePowder deliveryAntioxidantMaterials science
One embodiment of the invention is demineralized bone putty composition comprises: (1) demineralized bone matrix (DBM); and (2) a lipid fraction selected from the group consisting of lecithin and a mixture of lecithin and triglycerides containing unsaturated fatty acids. The putty composition is moldable, biocompatible, slowly resorbable, insoluble in tissue fluids, and non-extrudable. The composition delivers a biologically active product to animals and humans that will enhance bone formation at sites where bone is lost, deficient, or present in suboptimal amounts. The composition can further comprise calcium, an antioxidant such as Vitamin E or Vitamin C, or a hydrophilic polymer such as methylcellulose, a methylcellulose derivative, carboxymethyl cellulose, or hydroxypropyl methylcellulose. A second embodiment of the invention is a demineralized bone paste composition comprising: (1) about 15% to about 75% of an emulsion carrier, such as an aqueous phase; and (2) a bone-material-containing phase comprising: (a) demineralized bone matrix (DBM); and (b) an emulsifier component that is compatible with lipids. This bone paste composition is moldable, biocompatible, slowly resorbable, miscible with bone graft materials, soluble or partially soluble in tissue fluids, and extrudable.
Owner:BIOMET MFG CORP
Non-glycerol stabilized bone graft
ActiveUS8333985B2Slows proteolysisExtended shelf lifeBiocideOrganic active ingredientsProteinase activityGlycerol
A demineralized bone matrix (DBM) or other matrix composition is provided that has been stabilized by lowering the pH of the composition, reducing the water content, adding water substitutes, and / or increasing the amount of deuterated water present in the composition in order to reduce the activity of endogenous degrading enzymes such as proteases. A hydrated form of a stabilized DBM composition may be stable up to a year at room temperature at acidic pH. The acidified DBM compositions may be further stabilized by the addition of a stabilizing agent such as deuterated water, water substitutes, polymers, protease inhibitors, glycerol or hydrogels.
Owner:WARSAW ORTHOPEDIC INC
Nutritional supplement and use thereof
Nutritional supplements and methods for maintaining and / or improving the condition of bones or cartilage in a mammal, particularly a human. One such supplement comprises demineralized bone matrix (DBM) wherein the DBM comprises a bone growth improving amount of at least one osteoinductive growth factor. A preferred supplement composition further comprises at least one vitamin, such as vitamin E. One method comprises orally administering to the mammal on a periodic basis a supplement comprising DBM. In a preferred method the DBM composition is periodically administered and there is a further periodic administration of a therapeutically effective amount of a calcium-containing composition; the calcium-containing composition is administered temporally spaced apart from said DBM composition for maximum effectiveness.
Owner:ZYCAL BIOCEUTICALS HEALTHCARE COMPANY
Transplantable particulate bone composition having high osteoinductive capacity and methods for making and using same
ActiveUS20050152987A1Improved osteogenic capacityImproved osteoinductive activitySurgical adhesivesBone implantDemineralized bone matrixBone particle
A particulate bone of defined sizes which has a unique property of direct bone osteoinduction without going through endochondral ossification stage is disclosed. The bone is not subjected to chemical extraction or decalcification. This allows for the retention of all physiologically active components of native bone. The invention hinges on the newly discovered ability of bone particles of defined sizes to exert osteoinduction by a pathway superior to and distinct from demineralized bone matrix and similar preparations.
Owner:VIVEX BIOLOGICS GRP INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com