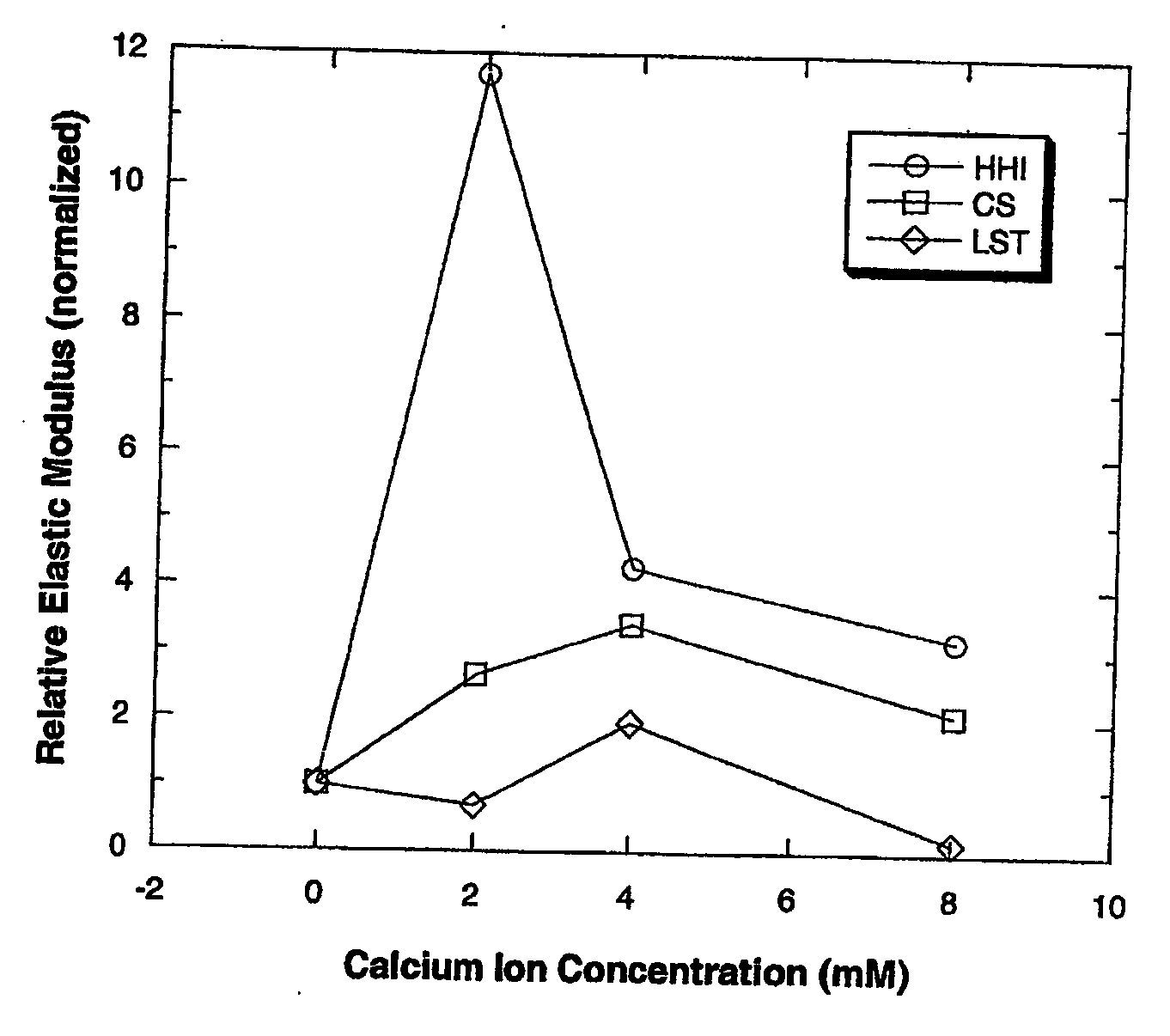
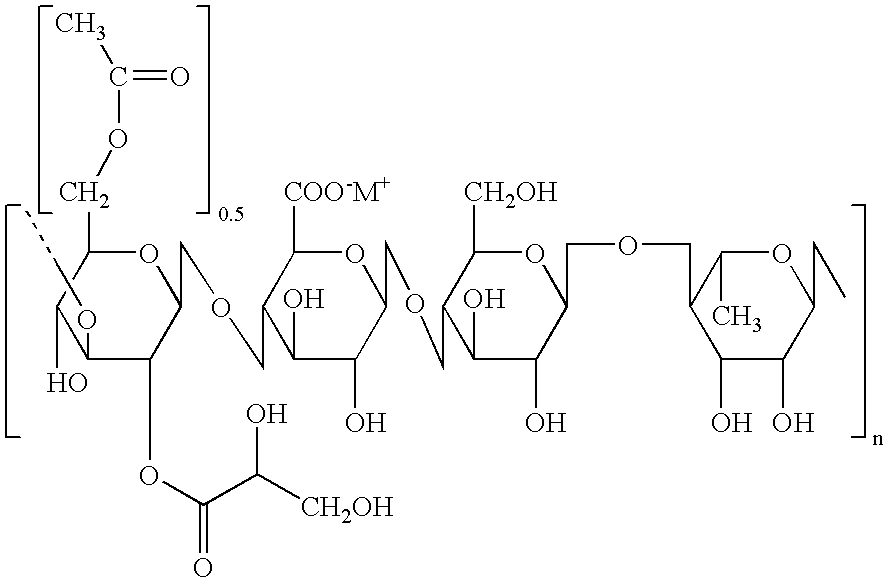
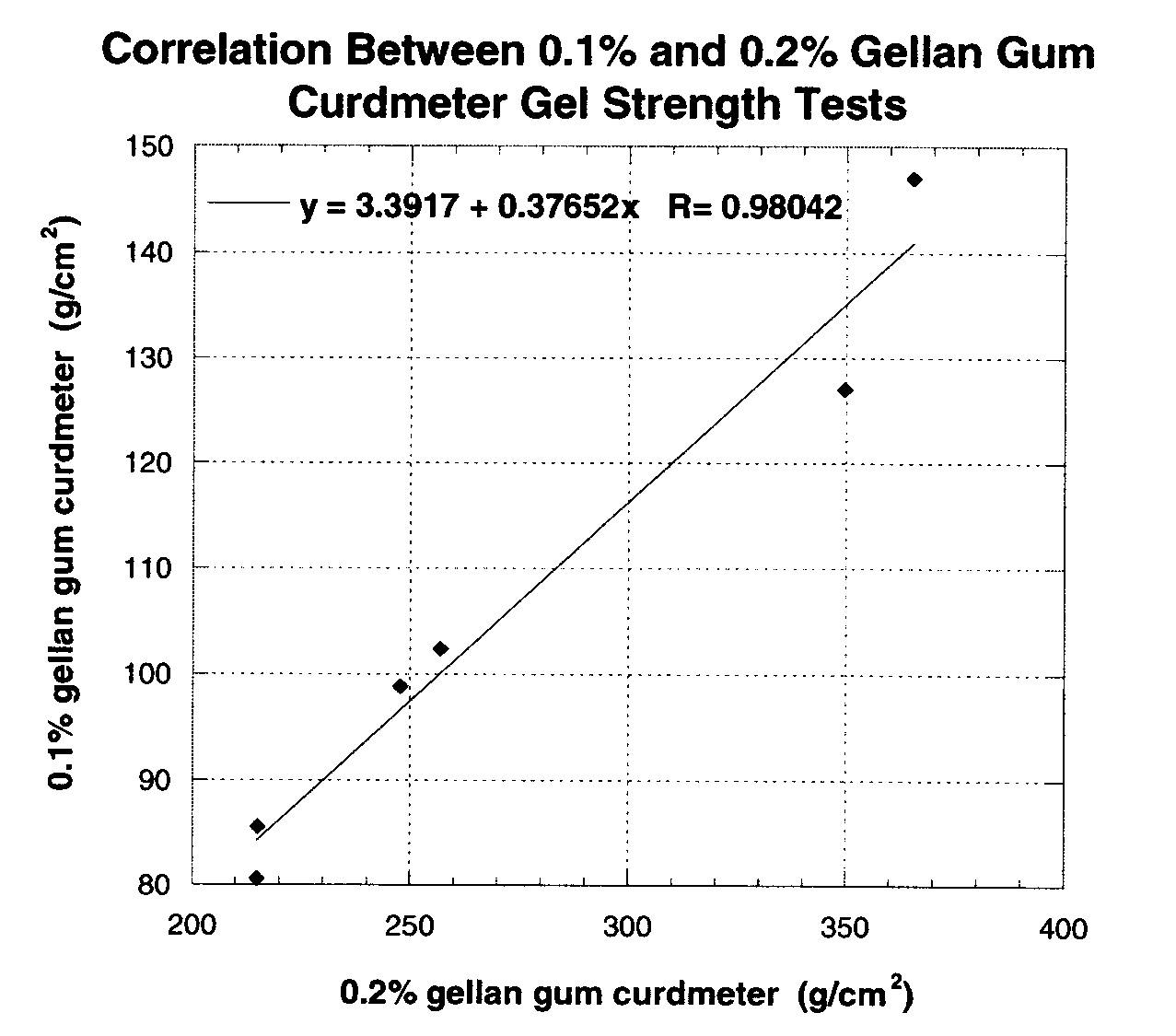
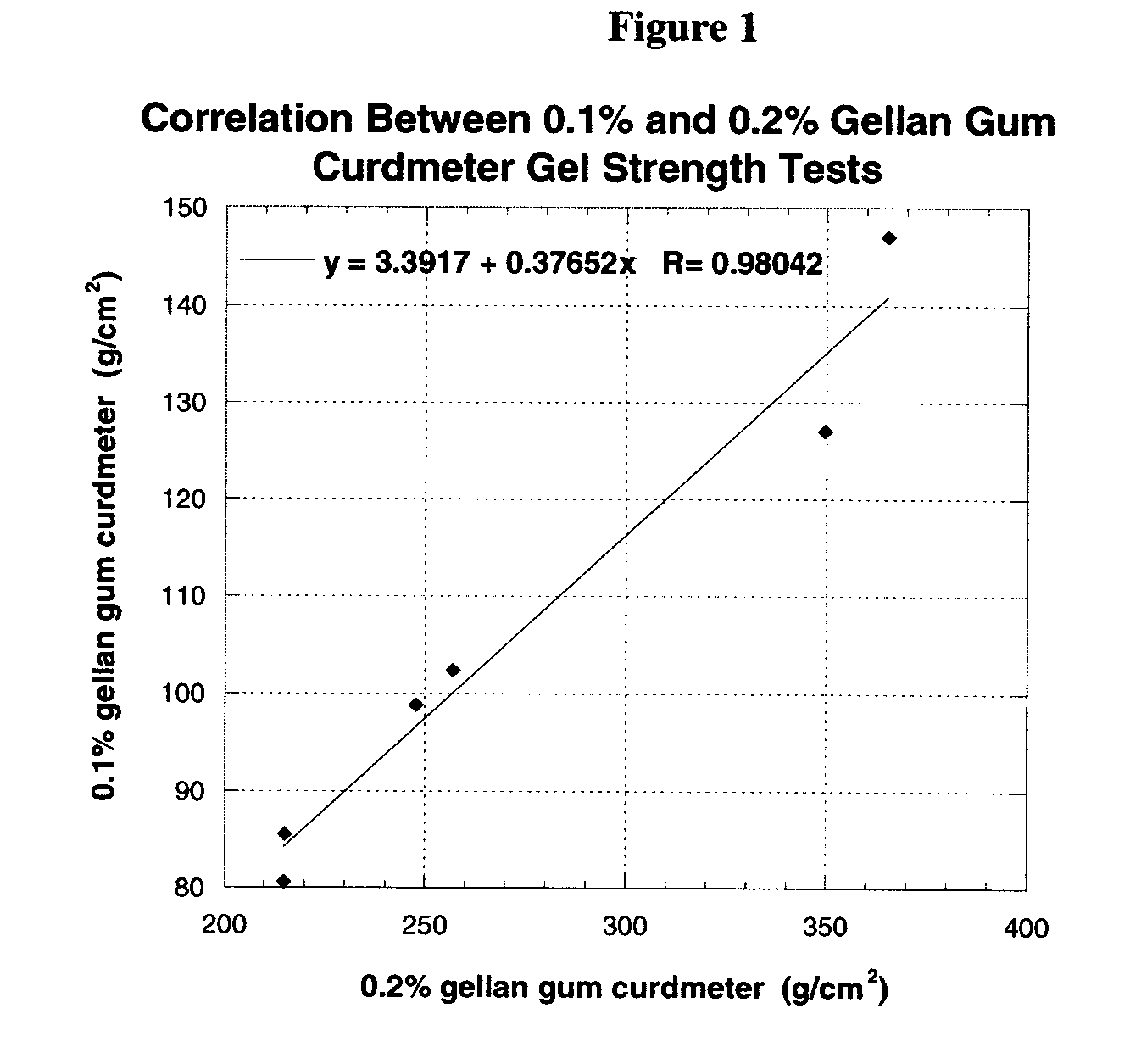
Patents
Literature
256 results about "Sphingomonas elodea" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
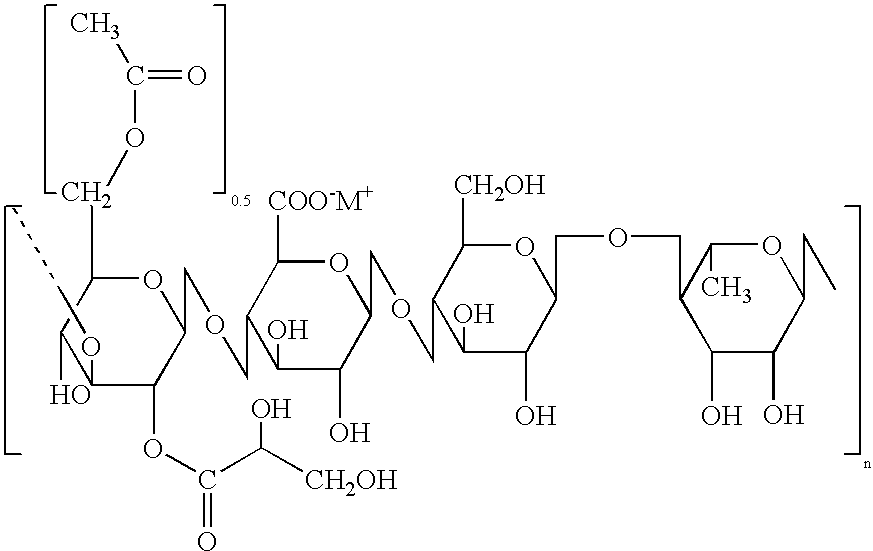
Gellan gum is a water-soluble anionic polysaccharide produced by the bacterium Sphingomonas elodea (formerly Pseudomonas elodea). The gellan-producing bacterium was discovered and isolated by the former Kelco Division of Merck & Company, Inc. in 1978 from the lily plant tissue from a natural pond in Pennsylvania, USA.
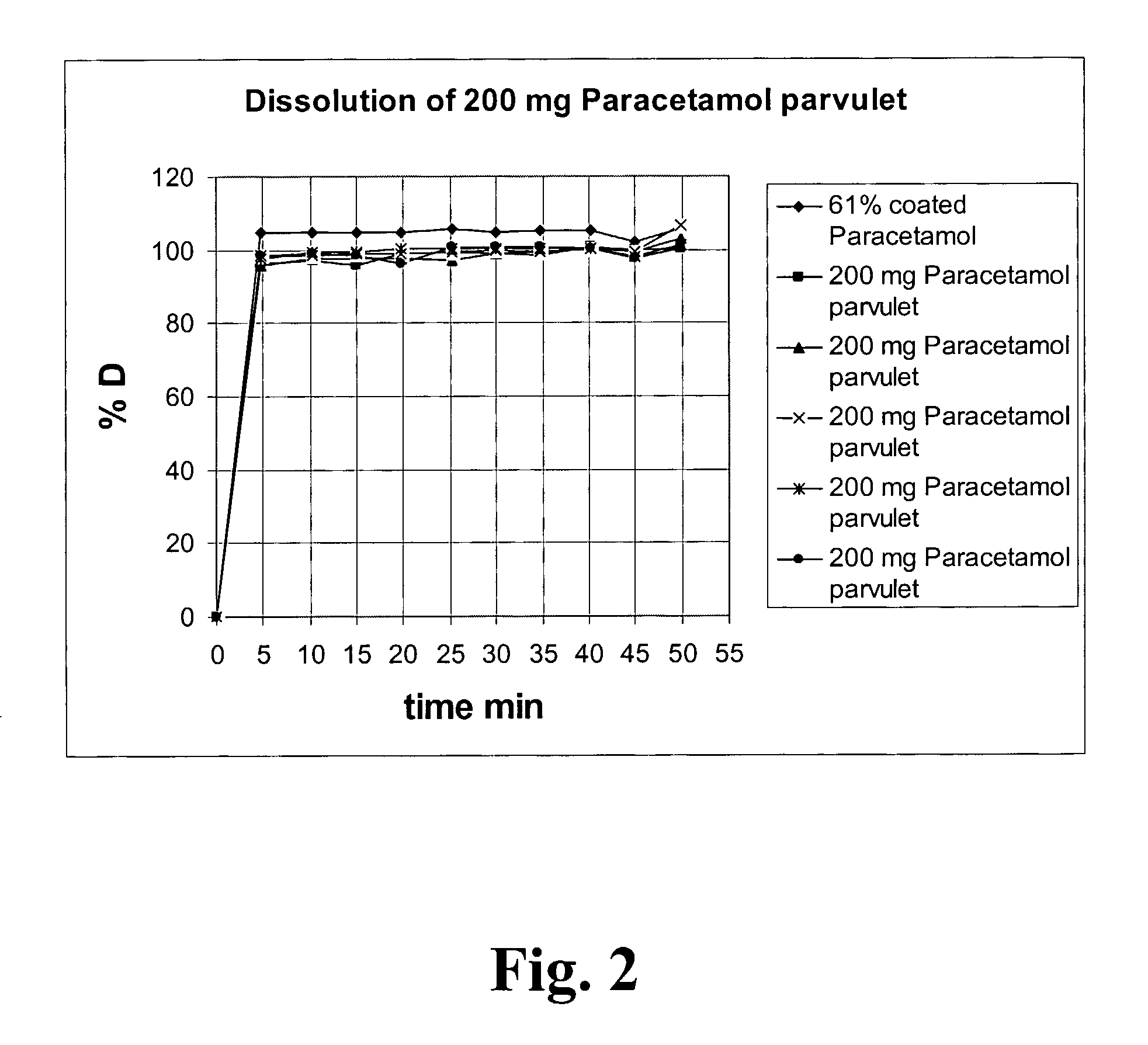
Swellable Dosage Form Comprising Gellan Gum
ActiveUS20080299199A1Easily can be orally ingestedIncrease intakeHeavy metal active ingredientsOrganic active ingredientsParticulatesGellan gum
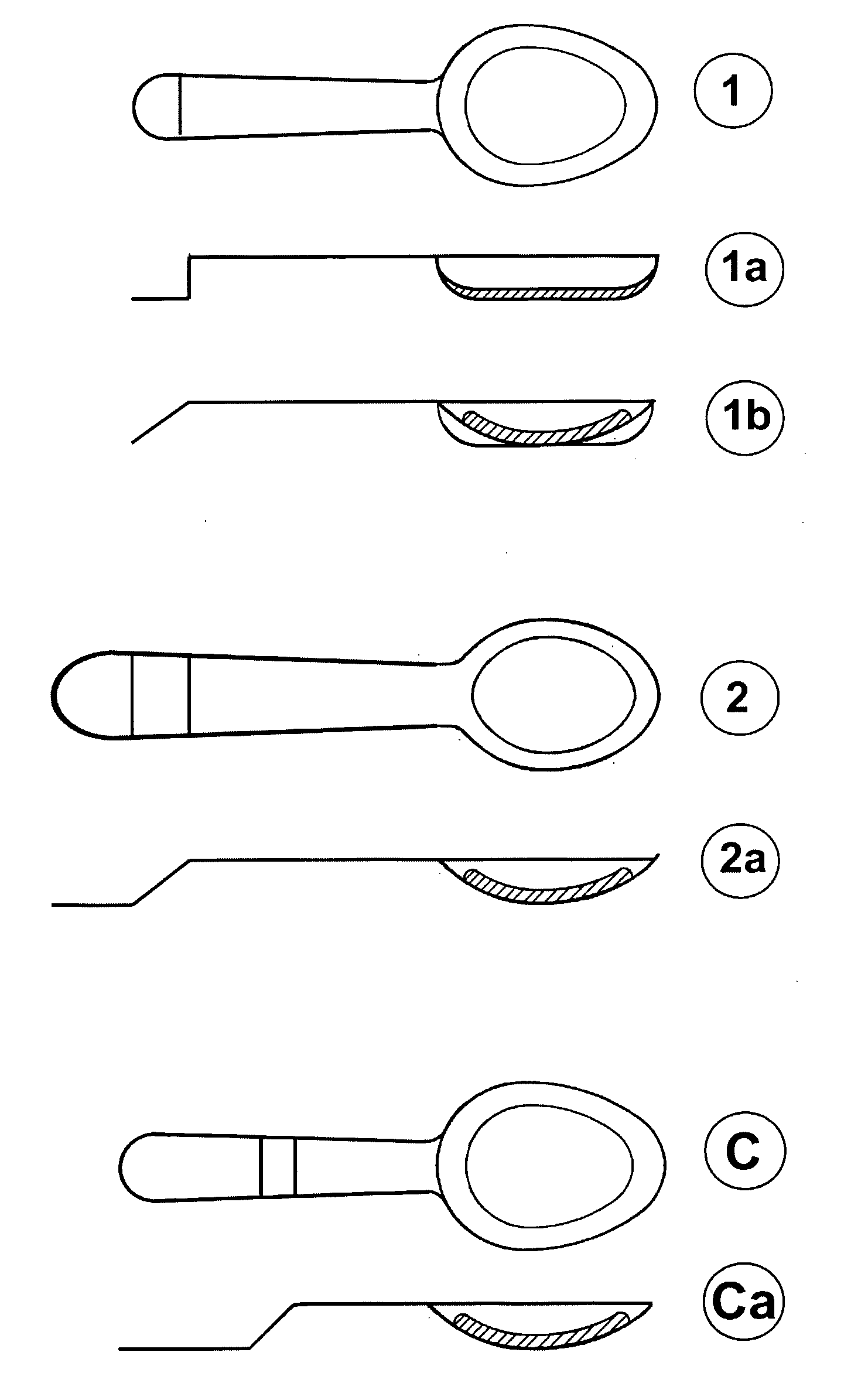
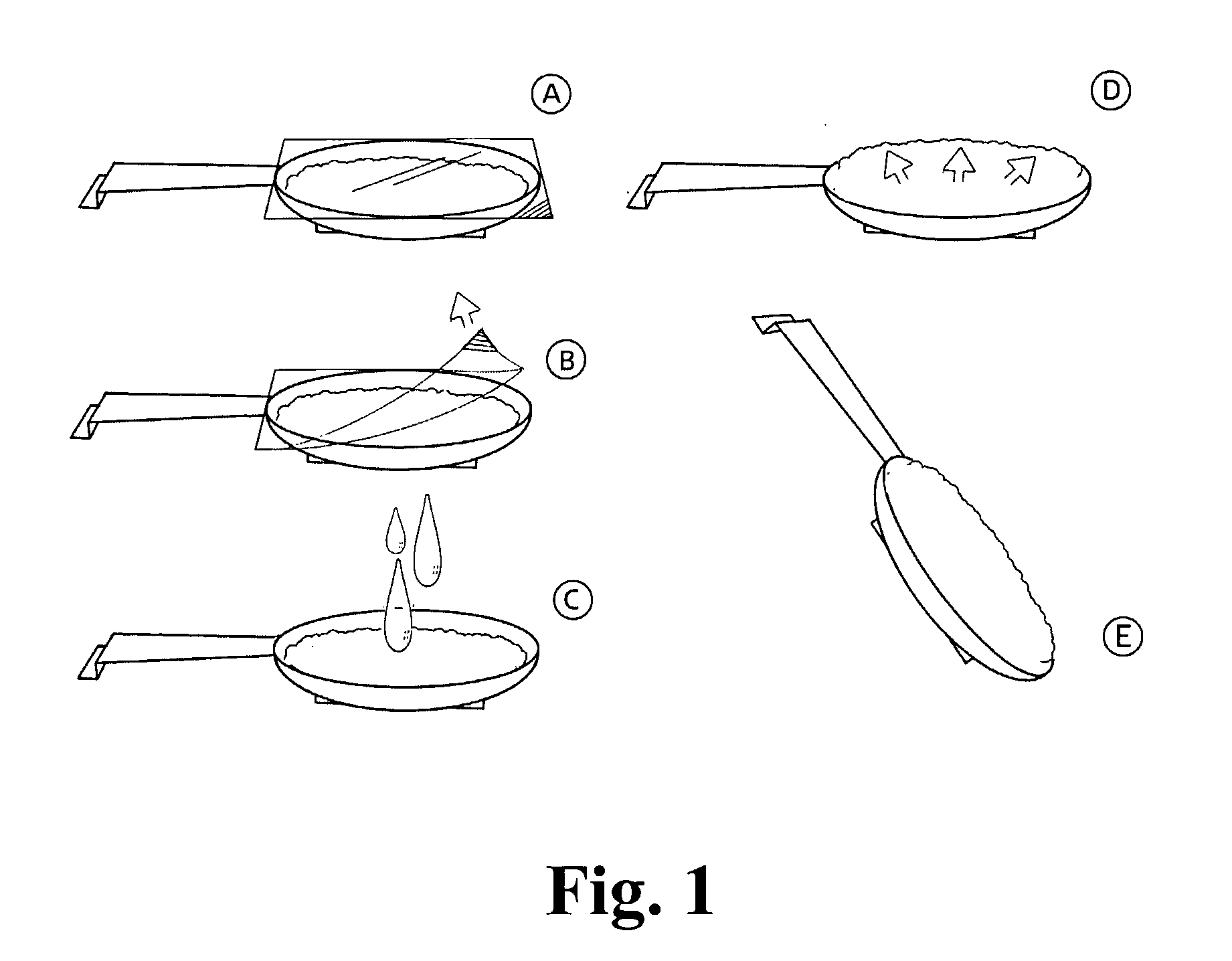
A novel dosage form. The dosage form is presented in particulate form and before oral ingestion the particulate material is subjected to an aqueous medium, whereby it is converted to a semi-solid form by swelling or gelling of one or more of the components, especially of a gellan gum, of the particulate matter. The invention also relates to a vehicle for oral administration of one or more active substances, the vehicle comprising a gellan gum arranged in a configuration allowing optimal water diffusion so that upon addition of a predetermined amount of an aqueous medium, without the necessity of applying shear forces or other mixing forces, within a time period of 5 minutes or less swells and / or gels and the texture of the swelled vehicle being similar to that of a soft pudding and having a viscosity of at least about 10,000 cps as measured by a Brookfield Viscometer with a #4 LV spindle at 6 rpm and at 20-25° C. In one embodiment of the invention, the particulate matter can be moulded into a desired shape or pressed onto a dispensing unit such as a spoon.
Owner:ADARE PHARM INC
Calcium stable high acyl gellan gum for enhanced colloidal stability in beverages
ActiveUS20050266138A1Improve colloidal stabilityGood particle suspensionSugar derivativesOther chemical processesGellan gumSphingomonas elodea
A low calcium sensitive (calcium stable) high acyl gellan gum is prepared for enhanced colloidal stability in beverages. The low calcium sensitive high acyl gellan gum has superior suspension performance for colloidal stability compared to other high acyl gellan gums. The low calcium sensitive high acyl gellan gum is prepared by adjusting the pH of a gellan fermentation broth (polymer solution) prior to pasteurization and reducing the pasteurization hold time compared to conventional pH levels and hold times.
Owner:CP KELCO U S INC
High Performance Gellan Gums and Methods for Production Thereof
The invention relates to high performance gellan gum compositions having a 0.1% curdmeter gel strength of at least about 117 g / cm2, i.e. from about 117 g / cm2 to about 400 g / cm2. The high performance gellan gums have a low acyl content but an increased molecular weight. One embodiment of the invention also relates to processes for producing high performance gellan gums having high clarity. The invention further relates to food and non-food industrial products comprising high performance gellan gums.
Owner:CP KELCO U S INC
Methods of making sterilized milk compositions comprising native gellan gum
InactiveUS6663911B2Optimize para-cresol reductionFunction increaseMilk preparationFood preservationGellan gumSphingomonas elodea
Owner:CP KELCO U S INC
Brownish lactobacillus beverage stabilizer and preparation method thereof, and sterilization-type brown lactobacillus beverage and preparation method thereof
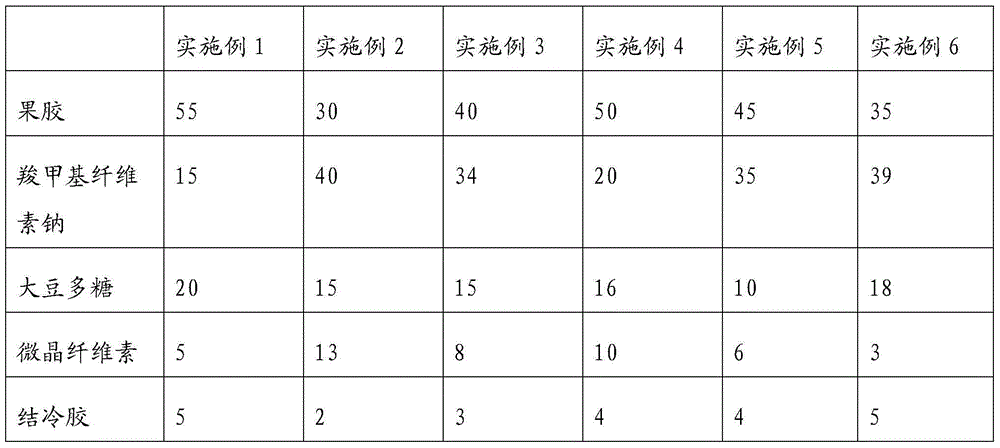
The present invention discloses a brownish lactobacillus beverage stabilizer and a preparation method thereof, and a sterilization-type brown lactobacillus beverage and a preparation method thereof. The stabilizer for the sterilization-type brown lactobacillus beverage includes the following components (weight percentages): 30 to 55% pectin, 15 to 40% sodium carboxymethylcellulose, 10 to 20% soybean polysaccharide, 5 to 13% microcrystalline cellulose, and 2-5% gellan gum. The stabilizer can be used in the preparation of the sterilization-type brown lactobacillus beverage. The stabilizer can effectively prevent certain negative phenomena such as water separating, precipitation, flocculation and stratification appeared in the beverage. During the six-month shelf life, the sterilization-type brown lactobacillus beverage is in uniform and stable state without precipitation and flocculation as well as significant stratification and with water bleeding less than 2.0 mm, and tastes fresh and full.
Owner:厦门欧凯科技有限公司
Microbial strains and processes for the manufacture of biomaterials
InactiveUS20090226962A1Increase enzyme activityHigh activityBacteriaHydrolasesBiotechnologyCell culture media
DNA constructs and genetically engineered microbial strains constructed using these DNA constructs, which produce a nuclease enzyme with specificity for DNA and / or RNA, are provided. These strains secrete nuclease into the periplasm or growth medium in an amount effective to enhance productivity and / or recovery of polymer, and are particularly suited for use in high cell density fermentation processes. These constructs are useful for modifying microbial strains to improve production and recovery processes for polymers such as intracellular proteins, such as enzymes, growth factors, and cytokines; for producing polyhydroxyalkanoates; and for producing extracellular polysaccharides, such as xanthan gum, alginates, gellan gum, zooglan, hyaluronic acid and microbial cellulose.
Owner:CJ CHEILJEDANG CORP
Pharmaceutical composition for oral administration
Provided is a pharmaceutical composition for oral administration containing a 5-HT.3 receptor antagonist, which is suitable for self-medication because of good preservation stability, low synthesis, good uniformity and good external appearance, and good smoothness in throat, and easiness to be taken. Concretely, it is a jellied pharmaceutical composition for oral administration containing a 5-HT.3 receptor antagonist, a gelatinizing agent, and water; and having a pH of 7 or less. In particular, there is provided the pharmaceutical composition, where the gelatinizing agent is carrageenan, pectin, agar, alginic acid, sodium alginate, gelatin, manna, Kodak, konjakmannan, glucomannan, chitosan, xanthan gum, tamarind seed polysaccharide, gellan gum, karaya gum, or cassia gum, or preferably the pharmaceutical composition further containing a thickener.
Owner:NICHI IKO PHARMA FACTORY
Bone filler material
ActiveUS20100255115A1Facilitate re-hydrationPowder deliveryPeptide/protein ingredientsGellan gumSphingomonas elodea
Described are bone generation matrixes having an admixture of demineralized bone matrix (DBM) particles and / or bone chips in combination with at least one binding agent selected from the group consisting of alginate, lectin, pectin, gellan gum, starch, collagen and combinations thereof in an aqueous solvent, wherein the DBM particles and / or bone chips are present in an amount of at least 65% by dry weight and the ratio of the aqueous solvent to the dry weight of the DBM particles / bone chips and at least one binding agent is between about 1:1 to about 1:6.
Owner:WARSAW ORTHOPEDIC INC
Acidified Protein Beverages Containing Suspended Particulates and Methods of Making Same
InactiveUS20080008814A1Large capacityEasy to operateMilk preparationFood ingredientsCarboxymethyl celluloseGellan gum
The use of gellan gum in combination with carboxymethyl cellulose (cellulose gum) in acidified protein beverages is described. Acifidied protein beverages comprising a combination of cellulose gum and gellan gum and methods to prepare these beverages are also described.
Owner:CP KELCO U S INC
Swellable dosage form comprising gellan gum
ActiveUS8383154B2Increase intakeEasy to doPowder deliveryHeavy metal active ingredientsParticulatesGellan gum
A novel dosage form. The dosage form is presented in particulate form and before oral ingestion the particulate material is subjected to an aqueous medium, whereby it is converted to a semi-solid form by swelling or gelling of one or more of the components, especially of a gellan gum, of the particulate matter. The invention also relates to a vehicle for oral administration of one or more active substances, the vehicle comprising a gellan gum arranged in a configuration allowing optimal water diffusion so that upon addition of a predetermined amount of an aqueous medium, without the necessity of applying shear forces or other mixing forces, within a time period of 5 minutes or less swells and / or gels and the texture of the swelled vehicle being similar to that of a soft pudding and having a viscosity of at least about 10,000 cps as measured by a Brookfield Viscometer with a #4 LV spindle at 6 rpm and at 20-25° C. In one embodiment of the invention, the particulate matter can be molded into a desired shape or pressed onto a dispensing unit such as a spoon.
Owner:ADARE PHARM INC
Genetically purified gellan gum
Mutational inactivation of proteins involved in para-cresol production in certain milk products results in improved taste and odor. The undesirable para-cresol forms over time as a result of enzymes produced by the bacterium that produces gellan gum. Since the gellan is typically used in a relatively unpurified form, the enzymes are added to the milk along with the gellan. Inactivation of the enzymes is a genetic means of eliminating the enzymes without requiring any additional purification or processing.
Owner:CP KELCO U S INC
Method for extracting low-acyl gellan gum suitable for tissue culture medium
The invention relates to a method for extracting low-acyl gellan gum suitable for a tissue culture medium, which comprises the following steps of: acyl removal treatment and enzyme treatment of a gellan gum fermenting fluid, flocculation of the gellan gum in a low-acyl state by bivalent or multivalent metal cation, clarification treatment of a gellan gum solution, dehydration treatment of the gellan gum solution, decoloring and ion exchange treatment, addition of a chelator / acid system to chelate bivalent cation possibly added during using the gellan gum and relative stability keeping of pH value, drying, and pulverization. The invention also provides the low-acyl gellan gum prepared by the method; the low-acyl gellan gum has the characteristics of good product appearance, good transmittance and high gelatinization strength which include the following properties: the chrominance is more than 83 percent; the transmittance is over 90 percent; and simultaneously, the gelatinization strength is between 400 and 650 g / cm.
Owner:ZHEJIANG DSM ZHONGKEN BIOTECH
Blends of different acyl gellan gums and starch
The present invention relates to a method of producing a film forming blend of different acyl gellan gums with starch having similar textural and functional properties compared to gelatin. Films prepared using such blends have a high modulus and excellent strength and elongation. The present invention also relates to soft capsules prepared using such blends or films, which have good sealability.
Owner:CORN PROD DEV INC
Gellan gum gel-based ceramic gel injection molding method
InactiveCN103072182AEnvironmentally friendlyReduce dosageAuxillary shaping apparatusCeramic sinteringWater baths
The invention discloses a gellan gum gel-based ceramic gel injection molding method, which belongs to the technical field of inorganic non-metal ceramic. The method comprises the following steps of: mixing ceramic powder, a dispersant and water to prepare ceramic slurry; defoaming under vacuum and then preserving heat in a water bath of 50-70 DEG C for 10-20 minutes; meanwhile, heating mixture of gellan gum and water to 70-80 DEG C to prepare uniform and stable aqueous solution with mass fraction of 1-5 percent; and adding the gellan gum aqueous solution and the aqueous solution containing calcium ions or magnesium ions with mass fraction of 1-10 percent into the prepared ceramic slurry and stirring for 5-10 minutes; finally preparing a ceramic blank in an imperforated mold; and sintering the ceramic blank to obtain a compact ceramic sintered body. The method provided by the invention has the advantages of economy, environmental friendliness, easiness in operation and convenience in industrial production, and the prepared compact ceramic sintered body has superior performance and wide application.
Owner:TSINGHUA UNIV
Sphingomonas paucimobilis strain and application thereof
InactiveCN103421718AExpand sourceMeet the needs of industrial productionBacteriaMicroorganism based processesGellan gumBiotechnology
The invention discloses a sphingomonas paucimobilis strain and an application thereof. The sphingomonas paucimobilis strain is named as sphingomonas paucimobilis QHZJUJW, with the preservation number: CGMCC No.7783. The stain is endophyte derived and screened from seeds of wild microporous grass in Qinghai-Tibet Plateau, can be used for industrialized production of gellan gum and provides a new way for the industrialized production of the endophyte and gellan gum.
Owner:ZHEJIANG UNIV
Isothermal preparation of heat-resistant gellan gels with reduced syneresis
InactiveUS20090104141A1Other chemical processesSedimentation separationGellan gumSphingomonas elodea
A gel comprising a deacylated gellan gum, an effective amount of a sequestrant, an effective amount of a syneresis control agent, and a gelation inducer is described. Also methods for making the gel are disclosed including a method for forming heat-resistant gels comprising mixing deacylated gellan gum and xyloglucan; hydrating the blend; resting the hydrated blend until a gel forms. The gels can be used in a variety of applications such as air freshener gels.
Owner:CP KELCO U S INC
Novel composite edible gum for preparing fruit jelly and the produced fruit jelly
A Novel composite edible gum for preparing a fruit jelly includes gellan gum, carrageenin and konjak gum and also the salt for reinforcing the gum performance. The invention also provides an application and a producing method for the composite edible gum. Additionally, the invention provides the fruit jelly food containing the composite edible gum and a producing method for the fruit jelly food.
Owner:ZHEJIANG DSM ZHONGKEN BIOTECH
Post-extracting method for low-acyl clean-type gellan gum
The invention relates to a post-extracting method for low-acyl clean-type gellan gum, which comprises the following steps of: acyl removal treatment and enzyme treatment of gellan gum fermenting fluid, flocculation of the gellan gum in a low-acyl state by bivalent or multivalent metal cation, clarification treatment of gellan gum solution, dehydration treatment of the gellan gum solution; decoloring and chelating treatment, drying, and pulverization. The invention also provides low-acyl gellan gum prepared by the method; the low-acyl gellan gum has the characteristics of good product appearance, high transmittance and high gelatinization strength, which are particularly shown as follows: the chrominance is more than 83 percent; the transmittance is over 85 percent; and simultaneously, the gelatinization strength is more than 1,000 g / cm.
Owner:ZHEJIANG DSM ZHONGKEN BIOTECH
Process for preparing microbiological polysaccharide Gellan gum
The invention relates to a preparation method of microbe polysaccharide Gellan gum belonging to the microbe fermentation production field. The method comprises steps of preparing the slope strains, preparing the seed liquid, inoculating the seed liquid into the fermentation culture medium to ferment. The invention solves the problems of high production cost, low product yield (the density of the Gellan gum coarse polysaccharide is 1.3g / 100ml) exiting in known technology. The invention employs starch as the carbon source for the second level expanding culture medium and fermentation culture medium, and has advantages of low cost, high yield. The density of the Gellan gum coarse polysaccharide is 1.5-1.6g / 100ml when the fermentation is finished.
Owner:HEBEI HENBO BIO TECH
Sphingomonaspaucimobilis of high-yield gellan gum and use therefor
InactiveCN1970738AReduce incubation timeShorten the fermentation cycleBacteriaFermentationGellan gumSphingomonas elodea
Owner:SHANDONG UNIV
Gellan gum post-extraction method
ActiveCN1635133AImprove safety and reliabilityReasonable process designFermentationGellan gumHypochlorite
The invention relates to a posterior extraction method of Gellan gum belonging to the microbe fermentation production field. The method comprises steps of pretreatment of the fermentation liquor, deacylation, filtration, extraction of the flocculate, solid-liquid separation, drying and pulverization. The invention overcomes the shortcomings of high manufacturing cost, single variety existing in known technology. By the deacylation treatment with different added hypochlorite, Gellan gum with different acyl content can be obtained without destruction of the Gellan gum molecular structure to meet different need. The flocculant employs alkali metal or alkaline earth chloride to substitute for partial organic solvent to improve the production safe reliability.
Owner:HEBEI HENBO BIO TECH
Gellan gum without organic solvent and production technique thereof
InactiveCN101062957AResidue reductionHigh strengthCosmetic preparationsToilet preparationsGellan gumAlcohol
The invention relates to a new type jointing cold glue, productive technology and usage. This invention possesses low organic solvent residual quantity (such as alcohol residual quantity is not more than 200ppm) and high gel strength, which is fit for many sites.
Owner:上海慧源植物胶囊股份有限公司
Method of high post extraction of acyl Gellan
This invention relates to microorganism fermentation domain. It provides a post extraction method of acyl gellan. It consists of these steps: pretreatment of the fermentation solution, flocculent deposition; solid and liquid separation, dry, crush and so on. The invention resolves the problem of high acyl gellan gum, because it has been product by CPkeclo company of America, and its price is very high, characterized in that its cost is very low because of process is very simple, energy cost is low, material cost is low, and the quality reaches the FccIv standard of America.
Owner:HEBEI HENBO BIO TECH
Plant-based fermented yogurt stabilizer and application thereof
InactiveCN110074193AImprove water holding capacityMaintain textureMilk substitutesFood sciencePhosphateFermentation
The invention provides a plant-based fermented yogurt stabilizer and application thereof, and belongs to the technical field of processing of fermented yogurt. The plant-based fermented yogurt stabilizer is prepared by compounding hydroxy propyl distarch phosphate, acetylated distarch phosphate, xanthan gum, gelatin, pectin, gellan gum and agar. The plant-based fermented yogurt stabilizer is addedbefore fermentation, thus the stability of the structure of fermented soybean milk is maintained under low-temperature cold storage conditions, precipitation of whey is avoided, good water binding capacity and the texture and taste of the fermented soybean milk are maintained, the rough texture of ordinary fermented soybean milk is improved, the fermented soybean milk tastes delicate and smooth,and not only is special bean flavor of soybeans maintained, but also unpleasant beany flavor is removed.
Owner:郑州康晖食品科技有限公司
Liquid nucleotides/nucleosides-containing product
ActiveUS20110027391A1Reduce shear rateImprove palatabilityBiocideOrganic active ingredientsCelluloseGellan gum
The invention pertains to a liquid composition for preventing and / or treating memory decline and / or cognitive dysfunction, Alzheimer's, Parkinson's and / or dementia, said composition comprising: (i) at least 50 mg nucleoside and / or nucleotide per 100 ml; (ii) between 0.2 and 10 grams protein per 100 ml; (iii) between 0.05 and 3 wt. % of 5 thickener, based on total weight of the composition. The thickener is preferably selected from the group consisting of cellulose, xanthan gum, gellan gum, alginate, guar gum, locust bean gum, gum karaya, gum tragacanth, carrageenan, and mixtures thereof. The composition preferably has a loss factor tan δ between 0.1 and 100, as measured at any strain in the range of 1 100% at 0.1 Hz and 20° C. It is particularly found that a thickener selected from the group consisting of gellan gum, xanthan gum and cellulose greatly reduces sedimentation.
Owner:NUTRICIA
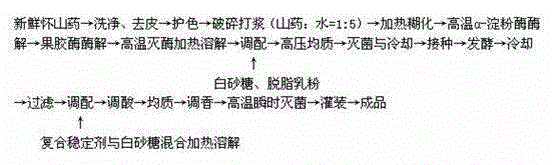
A multi-bacteria fermented Chinese yam beverage and a preparation method thereof
The present invention relates to a fermented beverage, and provides a multi-bacteria fermented Chinese yam beverage and a preparation method thereof against the present situation that the existing fermented yam beverage has long production cycle and poor stability and taste. The beverage is made from the following ingredients: Chinese yam, skim milk powder, white sugar, citric acid, sodium citrate, carboxymethylcellulose sodium, pectin, xanthan gum, gellan gum, 2,3-Dihydroxypropyl octadecanoate, sucrose ester, sodium tripolyphosphate, nisin, ethyl maltol, beer yeast and lactobacillus plantarum. The beverage has a unique flavor and a high nutritional value.
Owner:河南省淼雨饮品股份有限公司
Gellan gum composition, preparation method thereof and application thereof
InactiveCN101658692AMeet operational requirementsReduced bolus resistancePharmaceutical non-active ingredientsProsthesisGellan gumSphingomonas elodea
The invention discloses a gellan gum composition, which is mainly prepared from a gellan gum with a high acetylation degree and / or a gellan gum with a low acetylation degree or a derivative thereof. The invention also provides a preparation method of the composition and a use method of the composition. The composition has a large amount of application serving as an injection device and / or implanted device and a medicament releasing system. The composition has the advantages that: by controlling the gelling temperature and gel elasticity of the composition, the composition can be injected in asolution state when injected to beatify so as to reduce the injection pushing resistance, and then is changed into a cohesive gel to play the corresponding role; and when prepared into a water-solublemedicinal gel, the composition can be easily and uniformly mixed by stirring in a solution state to form a gel, thereby reducing the difficulty of uniformly mixing the gel.
Owner:INST OF BIOPHARM OF SHANDONG PROVINCE
Protein silk preparation and applications of protein silk in cosmetics
InactiveCN103013137AImprove mechanical stabilityImprove toleranceCosmetic preparationsHair cosmeticsShower gelSuspending Agents
The present invention relates to a protein silk preparation method and applications of protein silk as a cosmetic additive in the field of cosmetics. According to the present invention, natural polymer materials are adopted as a carrier and a film forming material to wrap natural silk protein hydrolysate-silk peptide to prepare a silk-like product, and the silk-like product is added to various transparent cosmetics so as to increase beautifying effects, wherein the natural polymer materials adopted as the carrier and the film forming material are sodium alginate, gellan gum, carrageenan and locust bean gum, the transparent cosmetics comprise two classes, one class is an essence and gel system adopted carbomer as a suspending agent, the essence and gel system comprises an essence liquid, an essence original liquid, a massage cream, a jelly mask and the like, the other class is a surfactant system adopted SF-1 as a suspending agent, and the surfactant system comprises a transparent facial cleanser, a transparent shower gel, a transparent shampoo and the like.
Owner:于仁毅
Aloe pulp particle drink
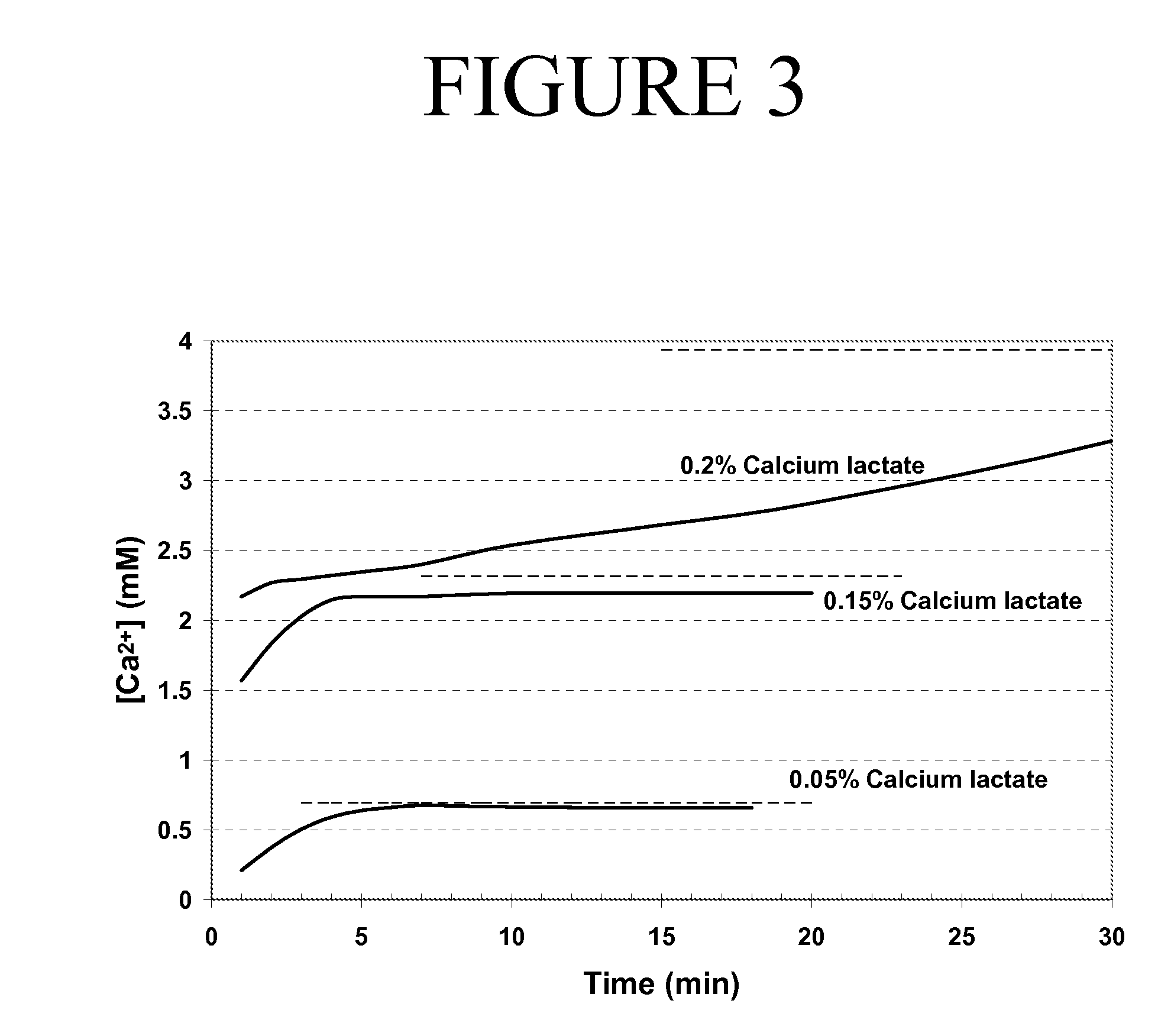
InactiveCN104172412AGood for healthHelps relieve symptomsMetabolism disorderDigestive systemCALCIUM LACTOBIONATEHigh pressure
The invention relates to the technical field of health drinks and in particular relates to an aloe pulp particle drink. The aloe pulp particle drink is characterized by comprising the following components by weight percent: 15% of aloe gel pudding, 10-16% of high fructose corn syrup, 0.03% of sodium carboxymethylcellulose, 0.1-0.15% of citric acid, 0.05% of calcium lactate, 0.02-0.04% of sodium citrate, 0.05% of gellan gum, 0.06% of edible essence and the balance of water. The aloe pulp particle drink has good health care effect on human digestive tract, is especially beneficial to relieving symptoms of patients with constipation and also has adjuvant therapy effect on the patients with high blood fat, high blood pressure, high blood sugar. By utilizing the aloe pulp particle drink, the health care effect of the aloe drink can be achieved after people drink the aloe pulp particle drink, and good mouthfeel of aloe gel pudding can be obtained.
Owner:山东上善堂生物工程有限公司
Curcumin microemulsion ion sensitive in situ gel preparation for intranasal administration and preparation method thereof
The invention discloses a curcumin microemulsion ion sensitive in situ gel preparation for intranasal administration, which uses curcumin as a raw medicine and adopts a surfactant, a cosurfactant, an oil phase and a gellan gum solution. According to the portions by weight, the preparation comprises 0.5-8 portions of curcumin, 4-25.6 portions of oil phase, 17-46.4 portions of surfactant, 17-46.4 portions of cosurfactant and 20-50 portions of gellan gum solution, wherein the mass concentration of the gellan gum solution is 0.1%-1.0%. The preparation method comprises the following steps of: firstly, uniformly mixing the oil phase, the surfactant and the cosurfactant in a mode of electromagnetic stirring, vortex oscillation or ultrasound; secondly, adding the curcumin, and fully stirring the mixture to be dissolved; thirdly, dropwise adding the gellan gum solution; and finally, continuously stirring the mixture to obtain the curcumin microemulsion ion sensitive in situ gel preparation for intranasal administration. After intranasal administration of the microemulsion ion sensitive in situ gel preparation disclosed by the invention, the medicine can reach brain targeted sites through a direct nose-brain path and a systemic circulation path, so that the curcumin has a treatment effect on brain tissues.
Owner:SHANDONG UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com