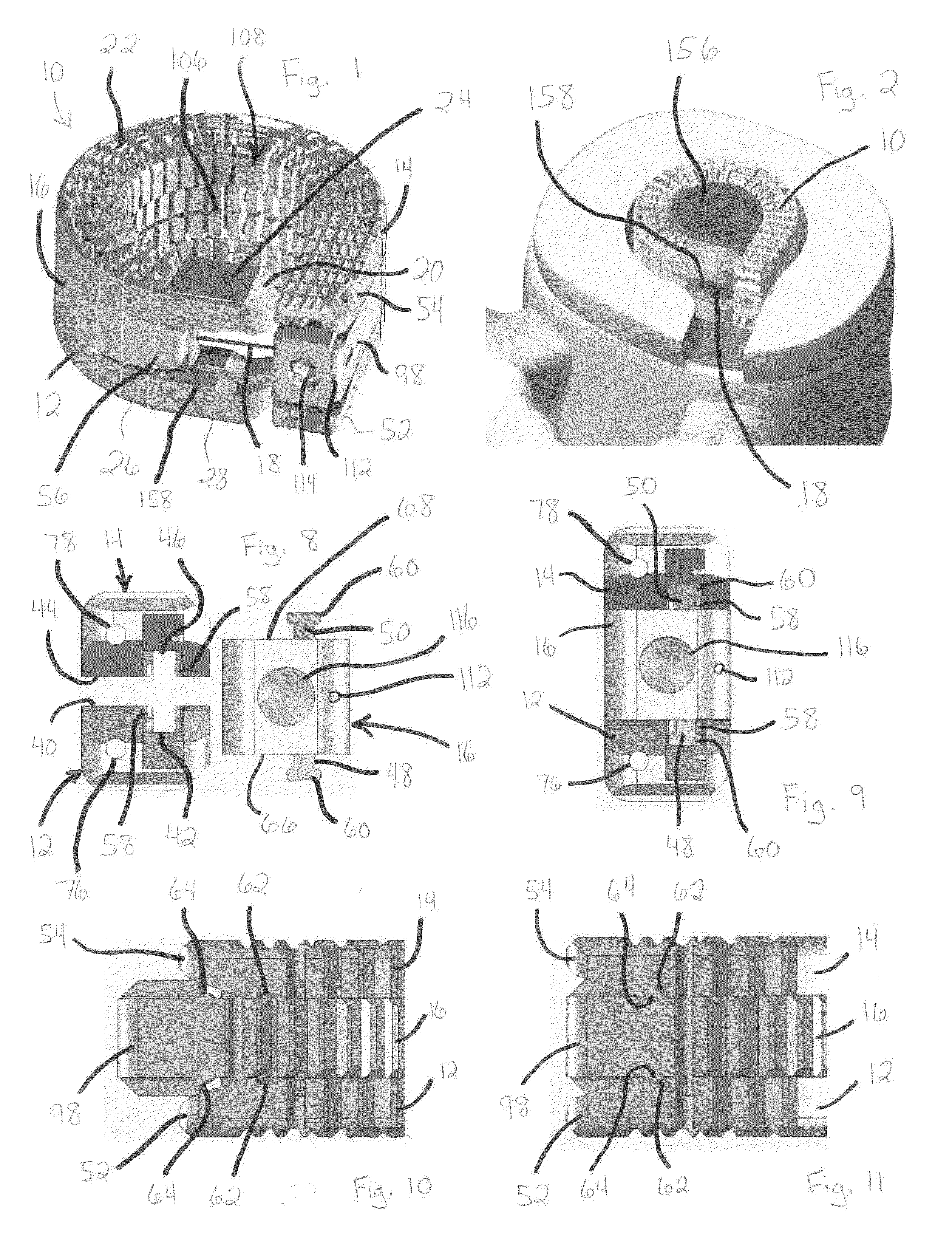
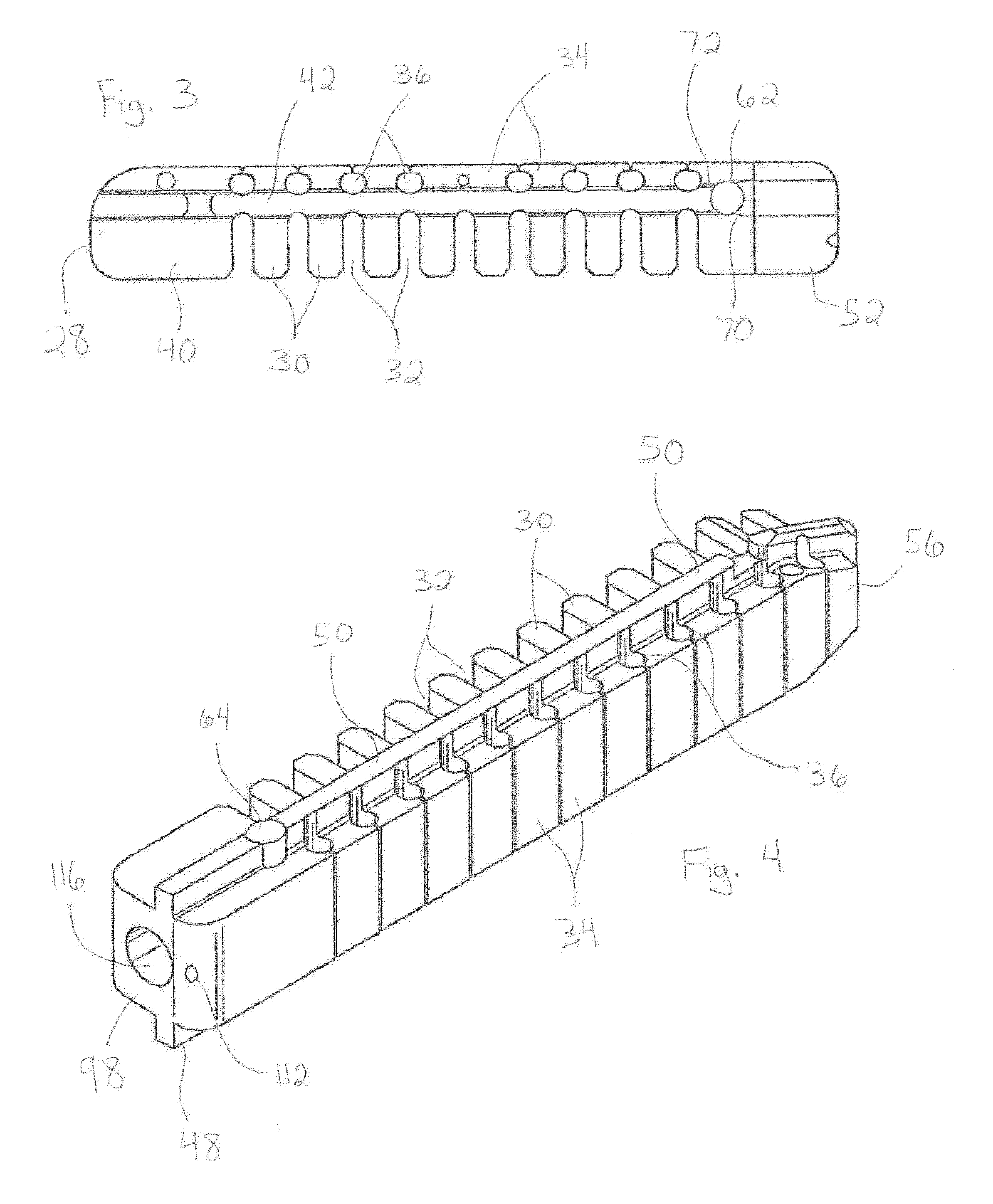
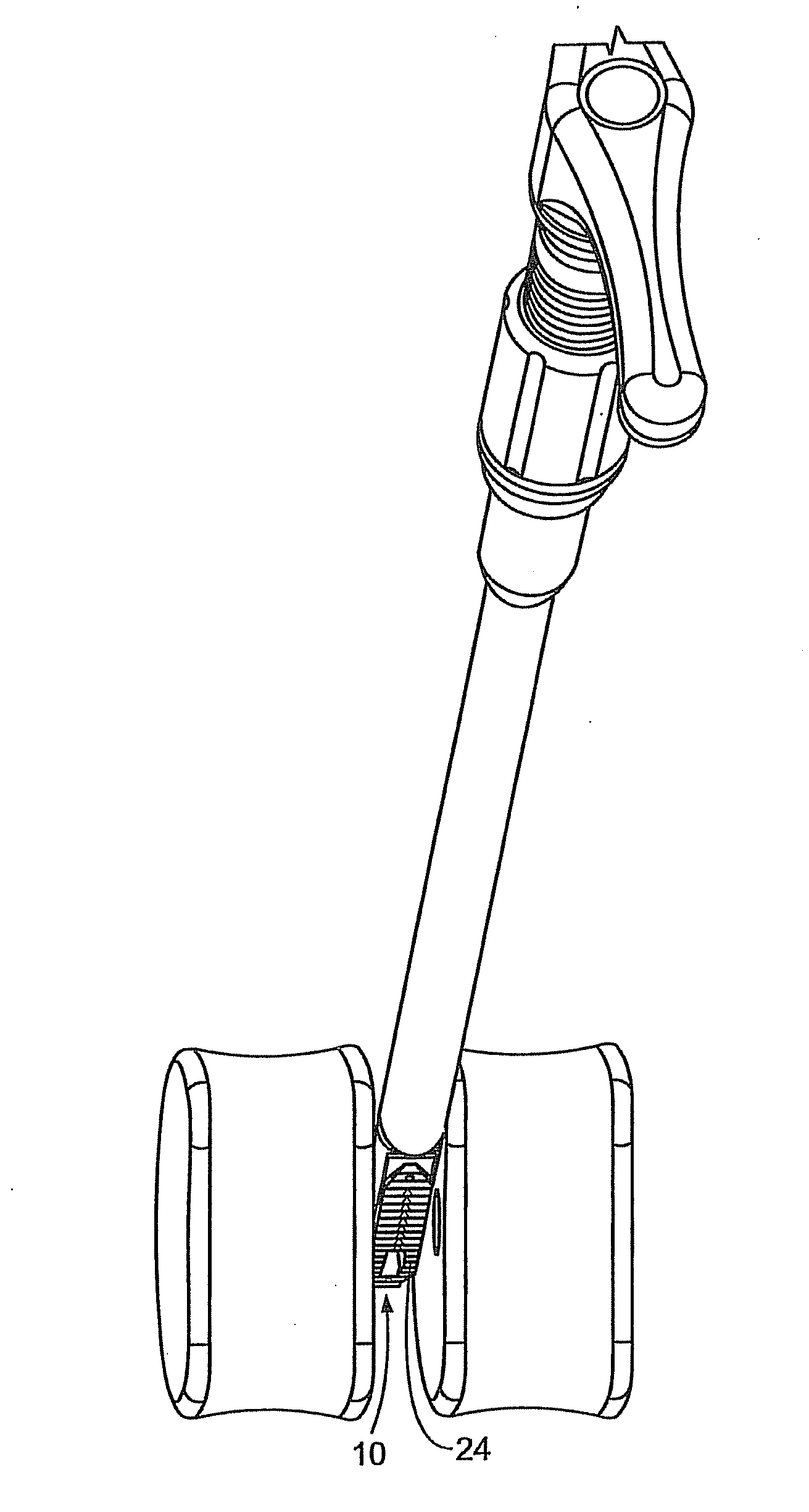
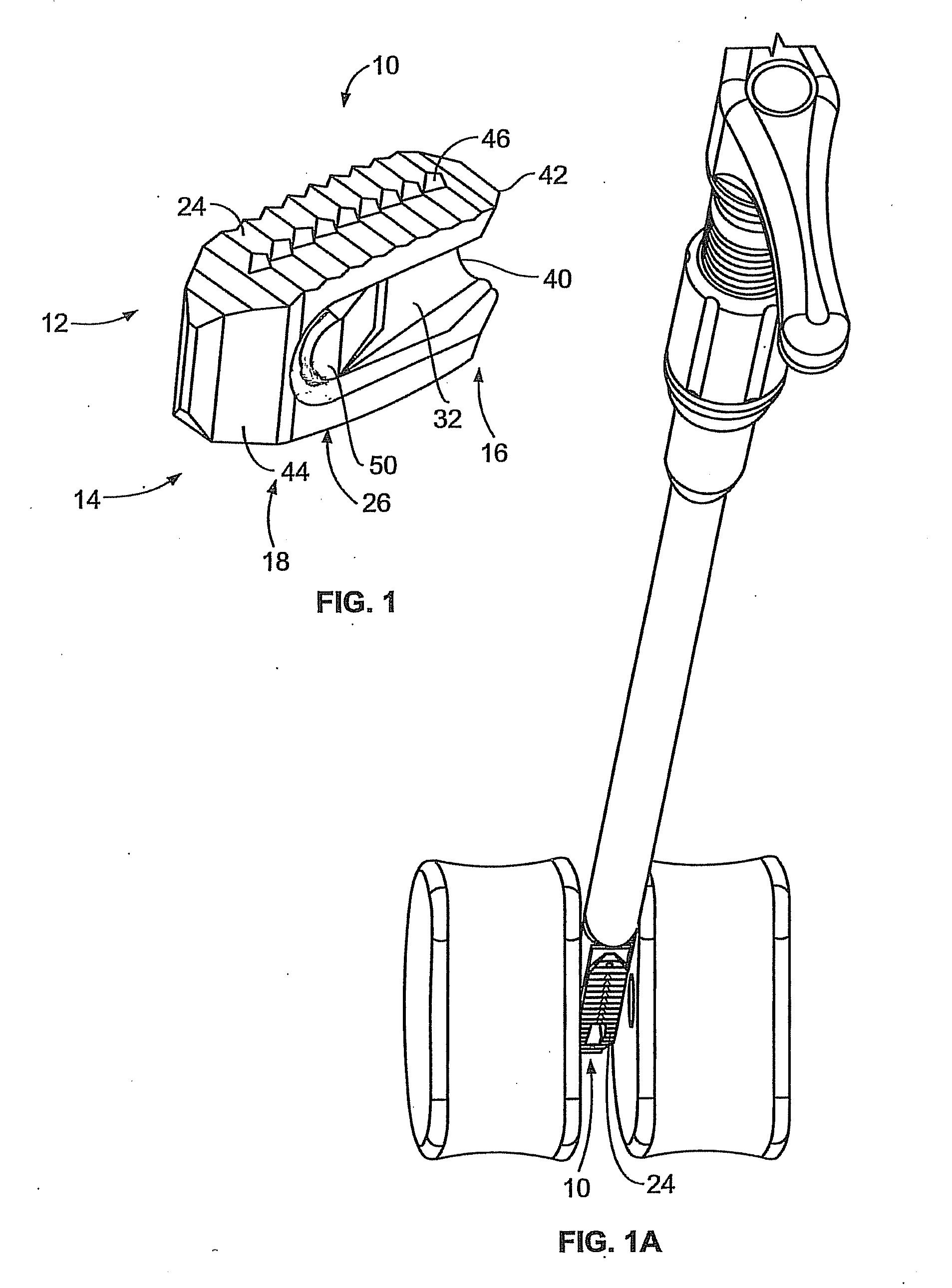
Patents
Literature
360 results about "Bone graft materials" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
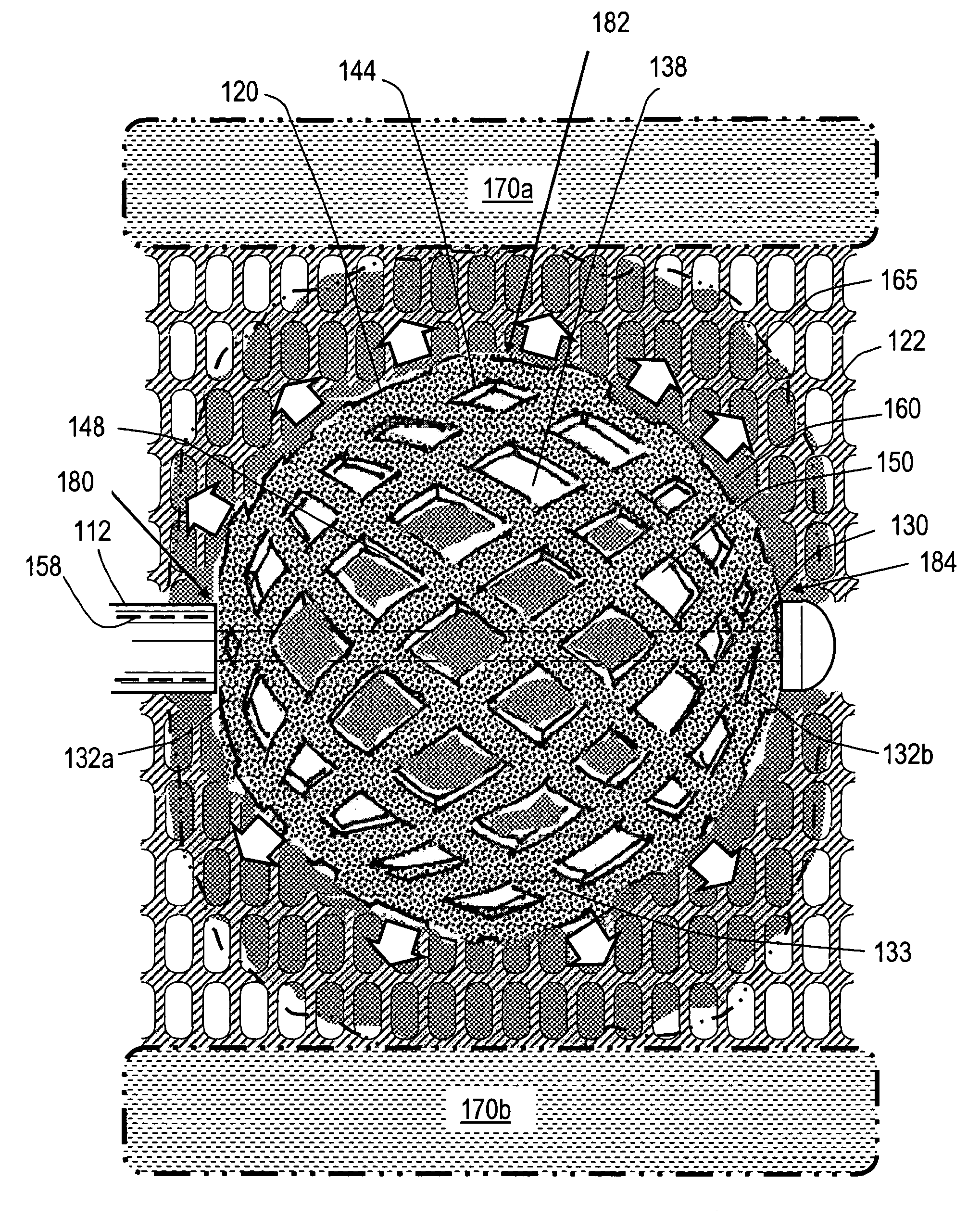
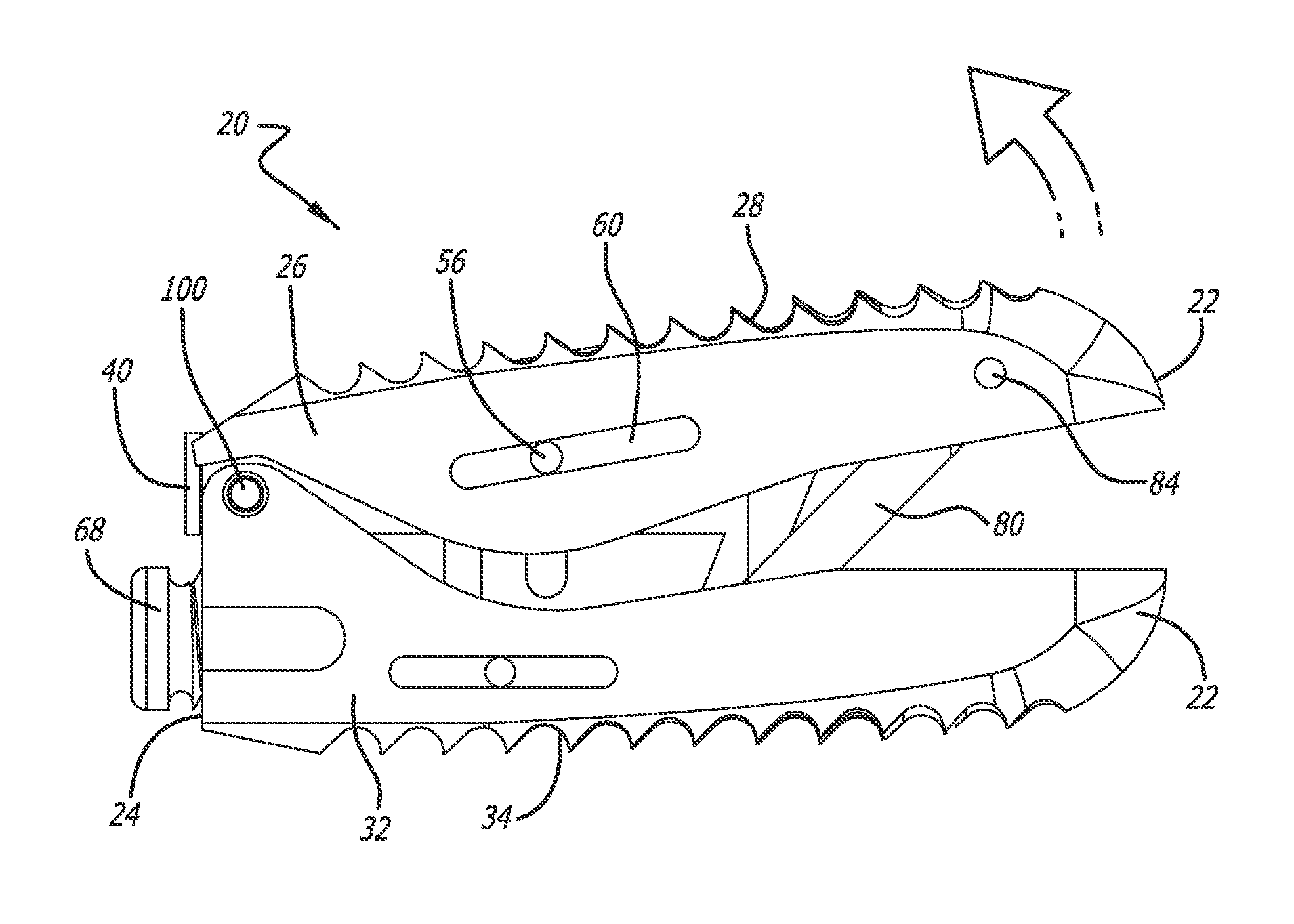
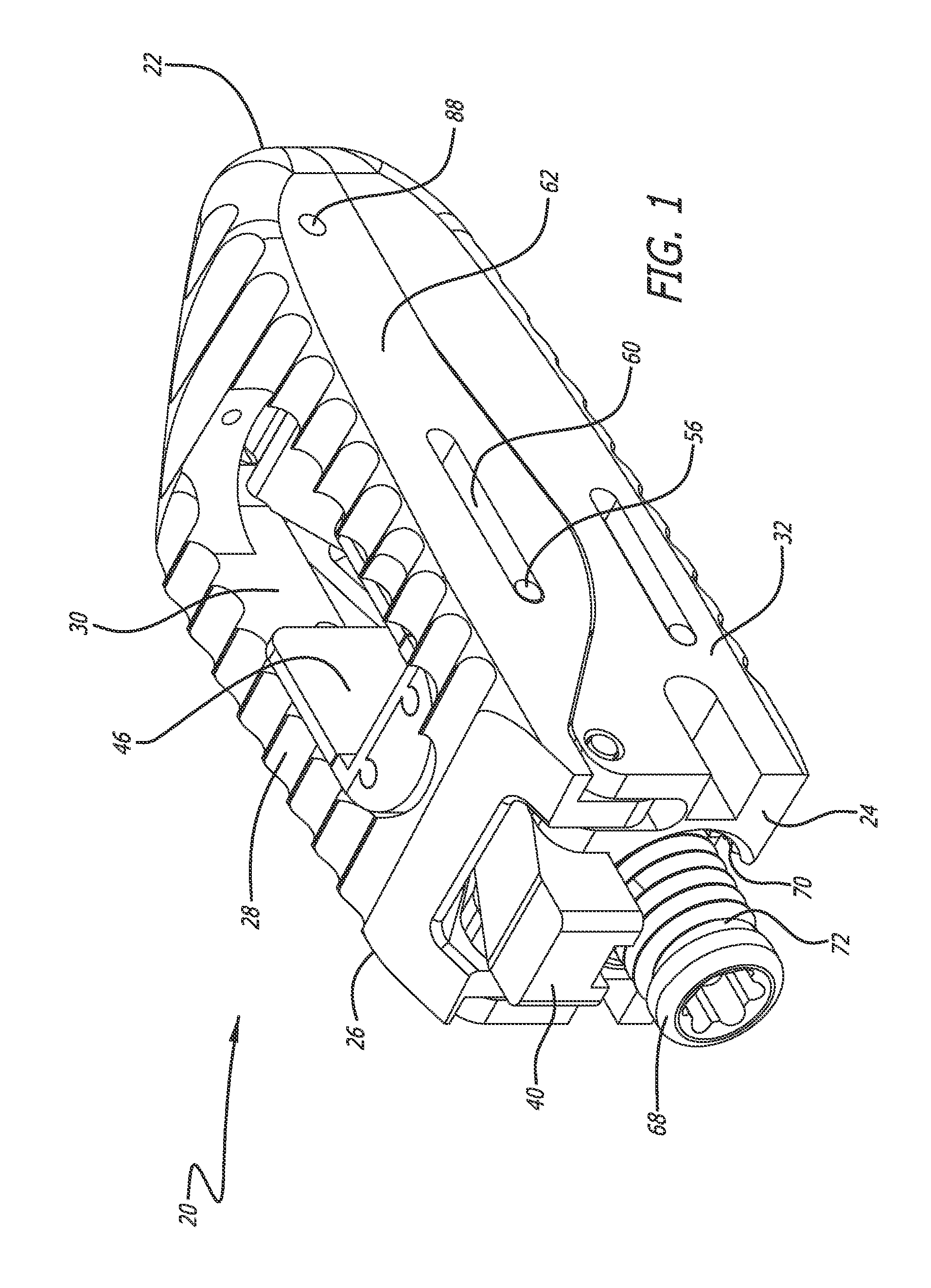
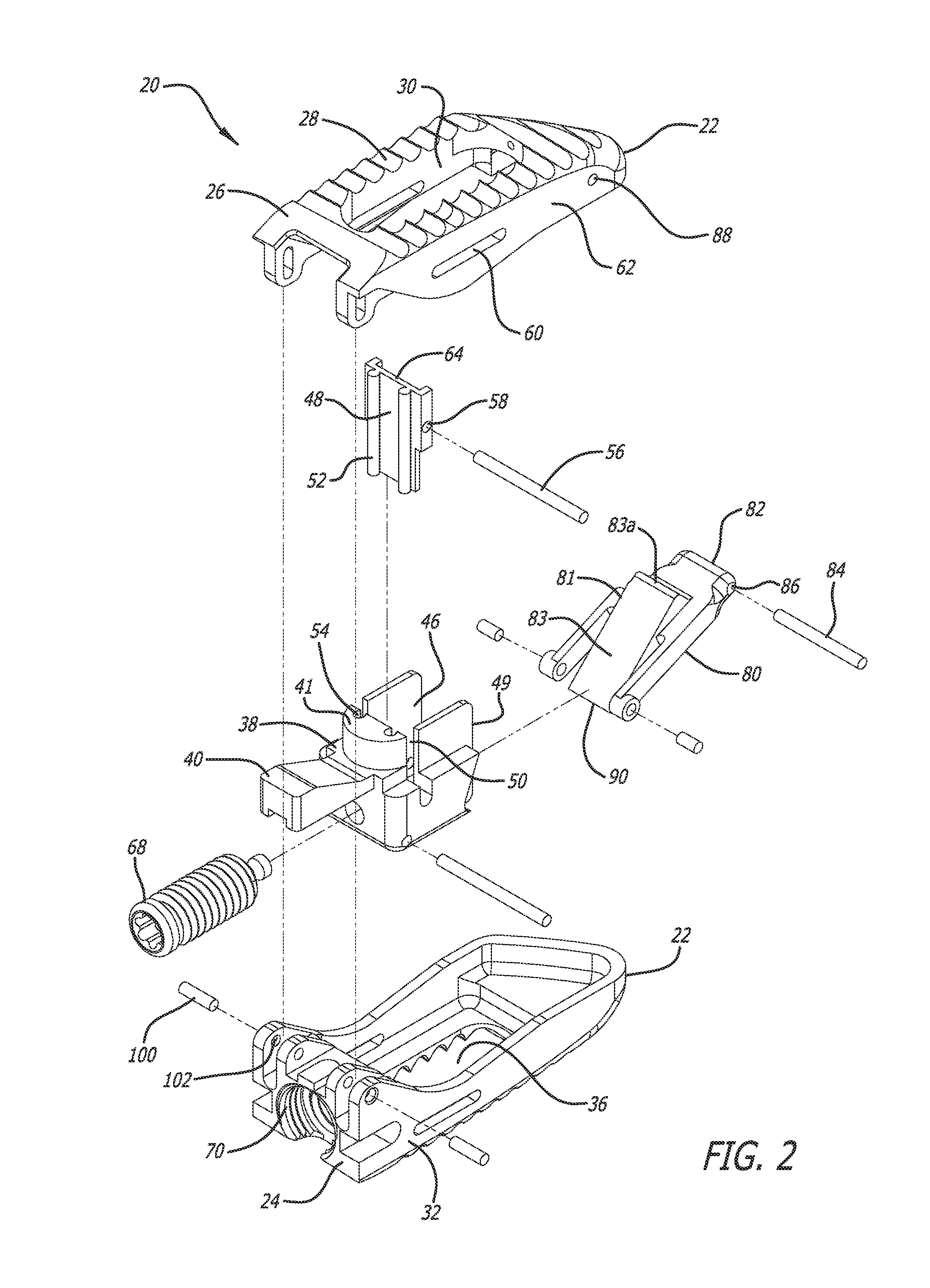
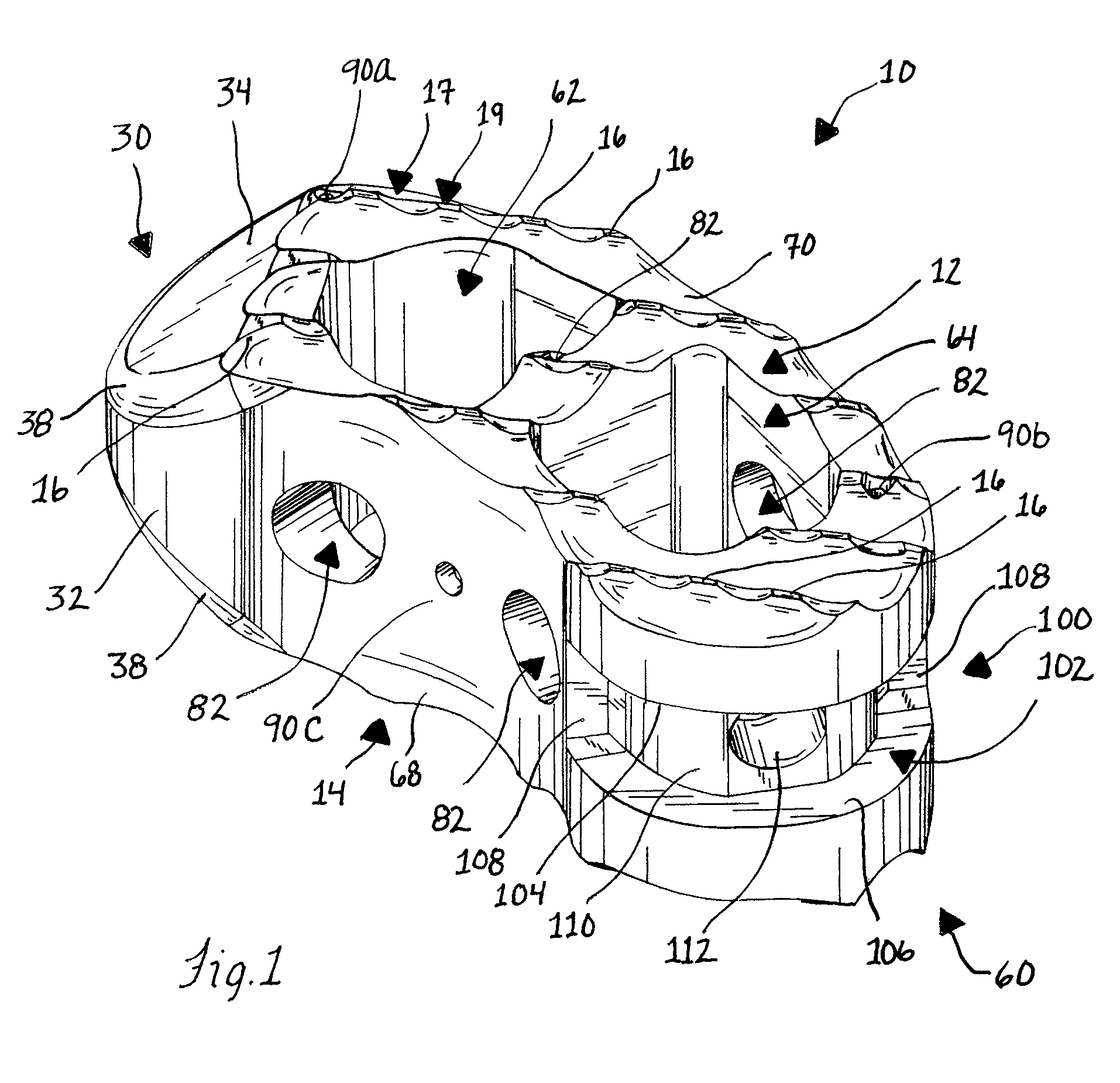
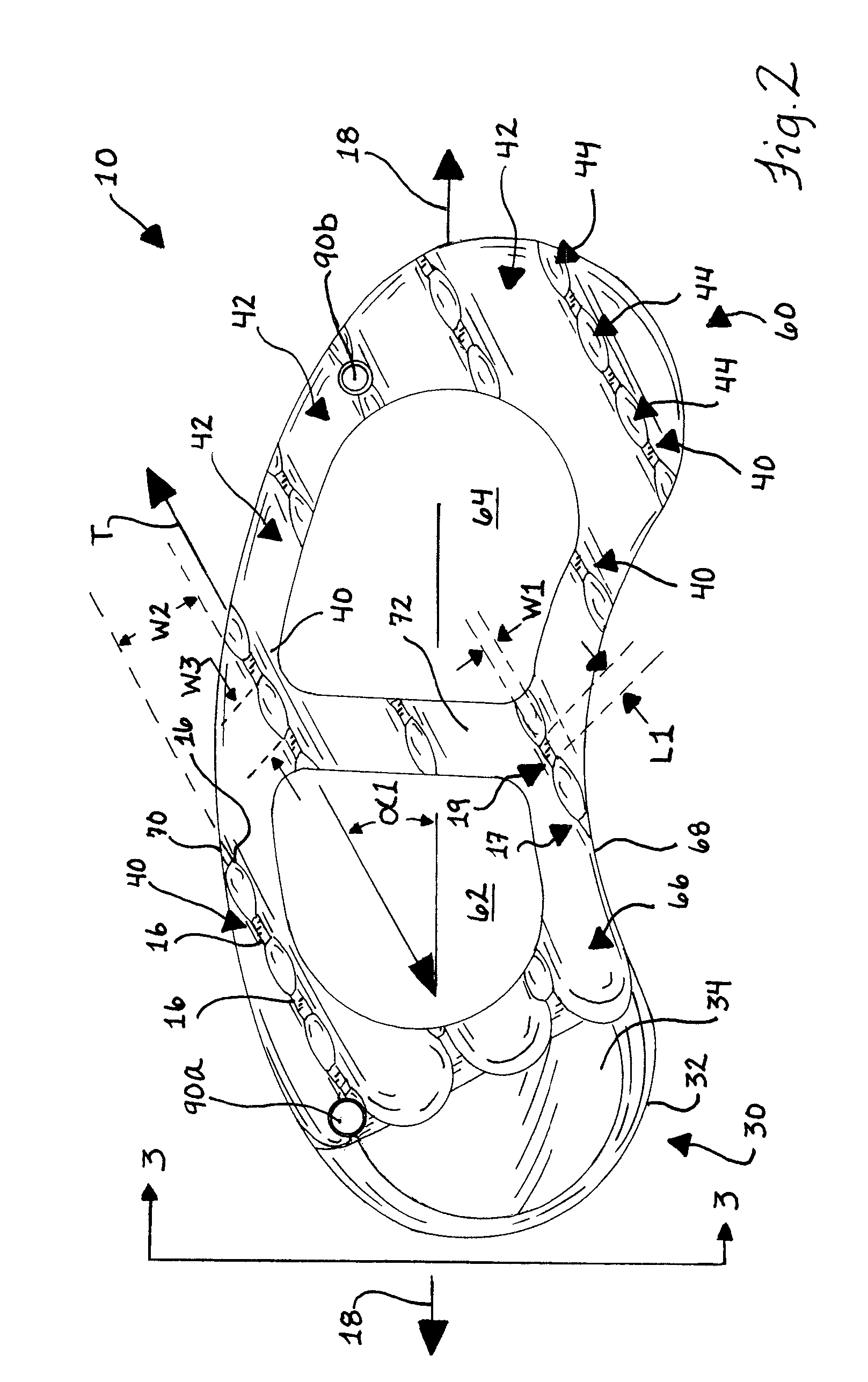
Stent systems and methods for spine treatment
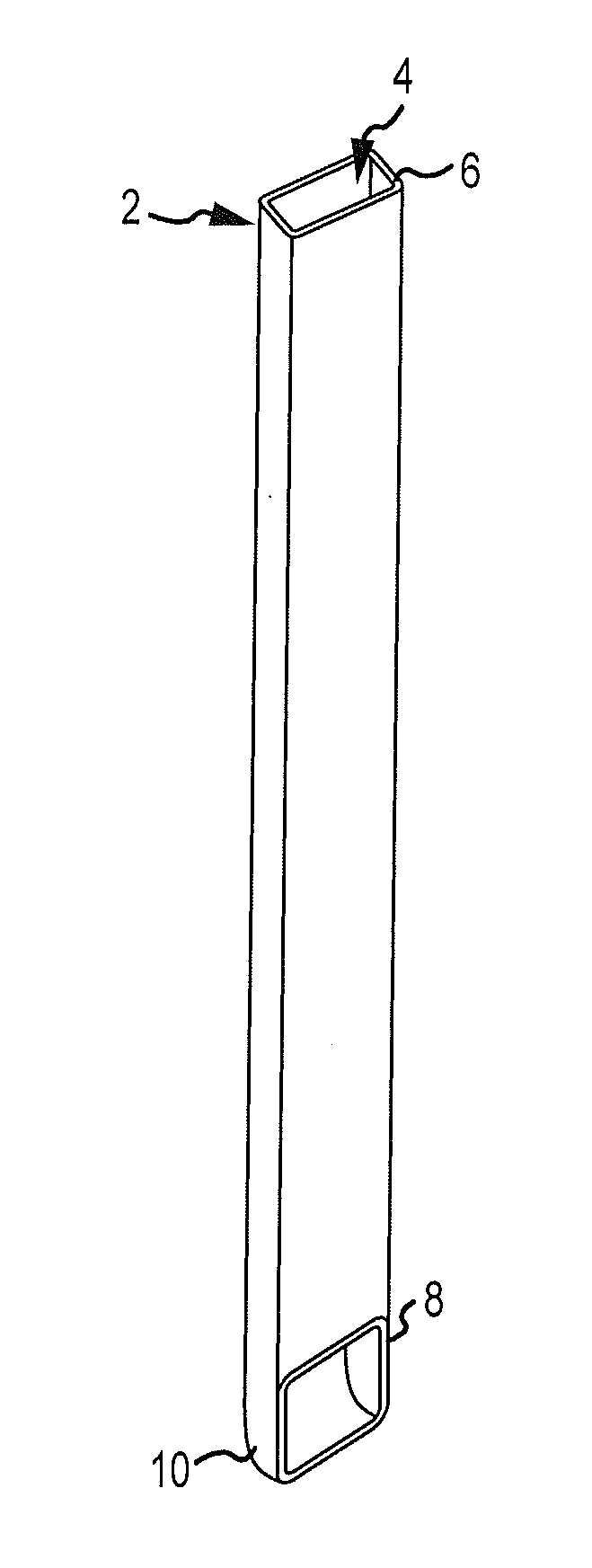
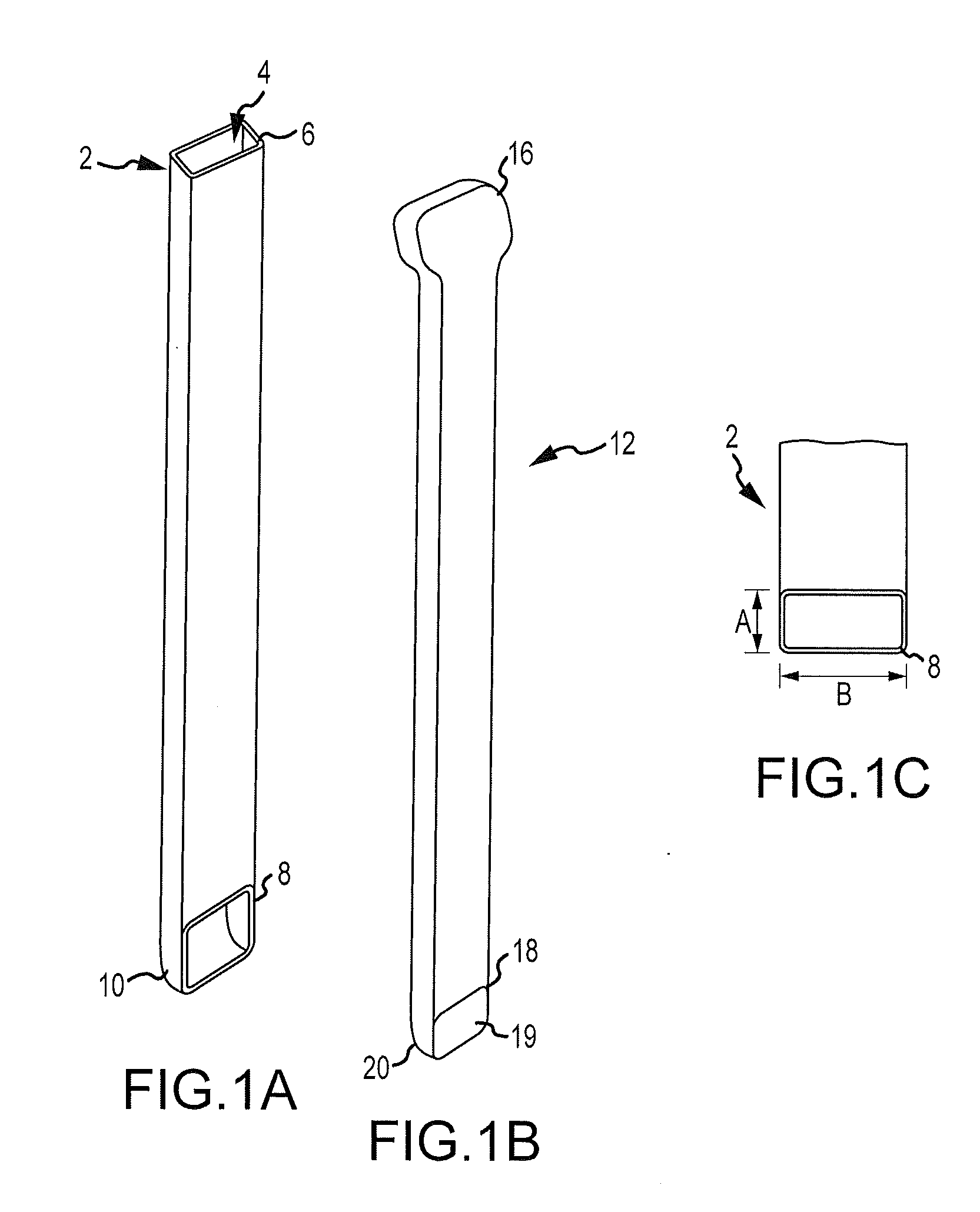
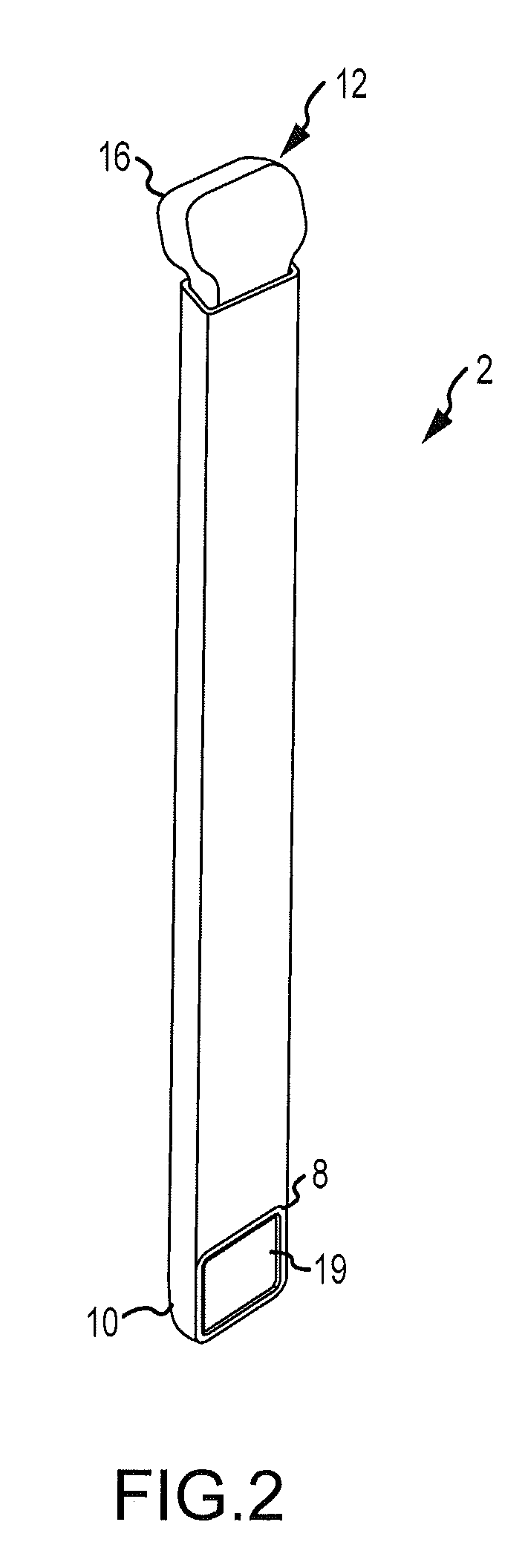
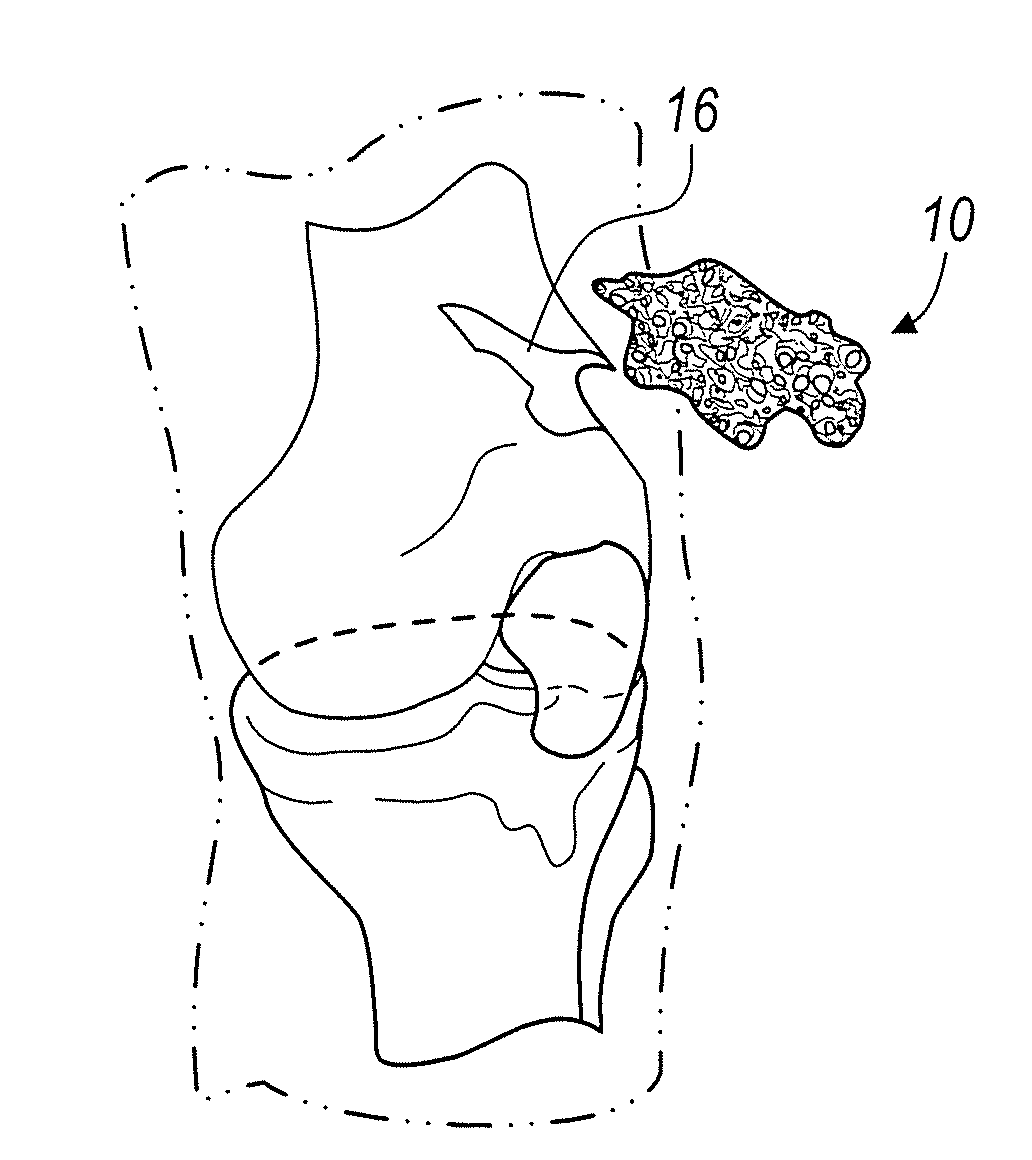
InactiveUS20060100706A1Prevent subsidenceRestore body heightInternal osteosythesisSpinal implantsSpinal columnCardiac allograft
Stent systems and methods for expanding and deploying stents in hard tissue such as bone, more particularly within a vertebral body. One exemplary method includes using a stent body that is coupled to a high speed rotational motor with the stent expandable and detachable from an introducer working end. In one embodiment, the stent is a deformable metal body with zig-zag type struts in an expanded configuration that carries diamond cutting particles bonded to the strut surfaces. The “spin” stent is rotated at high rpm's to remove cancellous bone from the deployment site together with irrigation and aspiration at the end of the probe that carries the stent. The stent may be expanded asymmetrically, such as with first and second balloons or by using an interior restraint, to apply vertical distraction forces to move apart the cortical endplates and support the vertebra in the distracted condition. The cancellous bone about the expanded stent as well as the interior of the stent can be filled with a bone cement, allograft or other bone graft material. In one method of use, the spin stent is designed and adapted for (i) treating a vertebral compression fracture (VCF) or for (ii) reinforcing an osteoporotic vertebral body.
Owner:DFINE INC
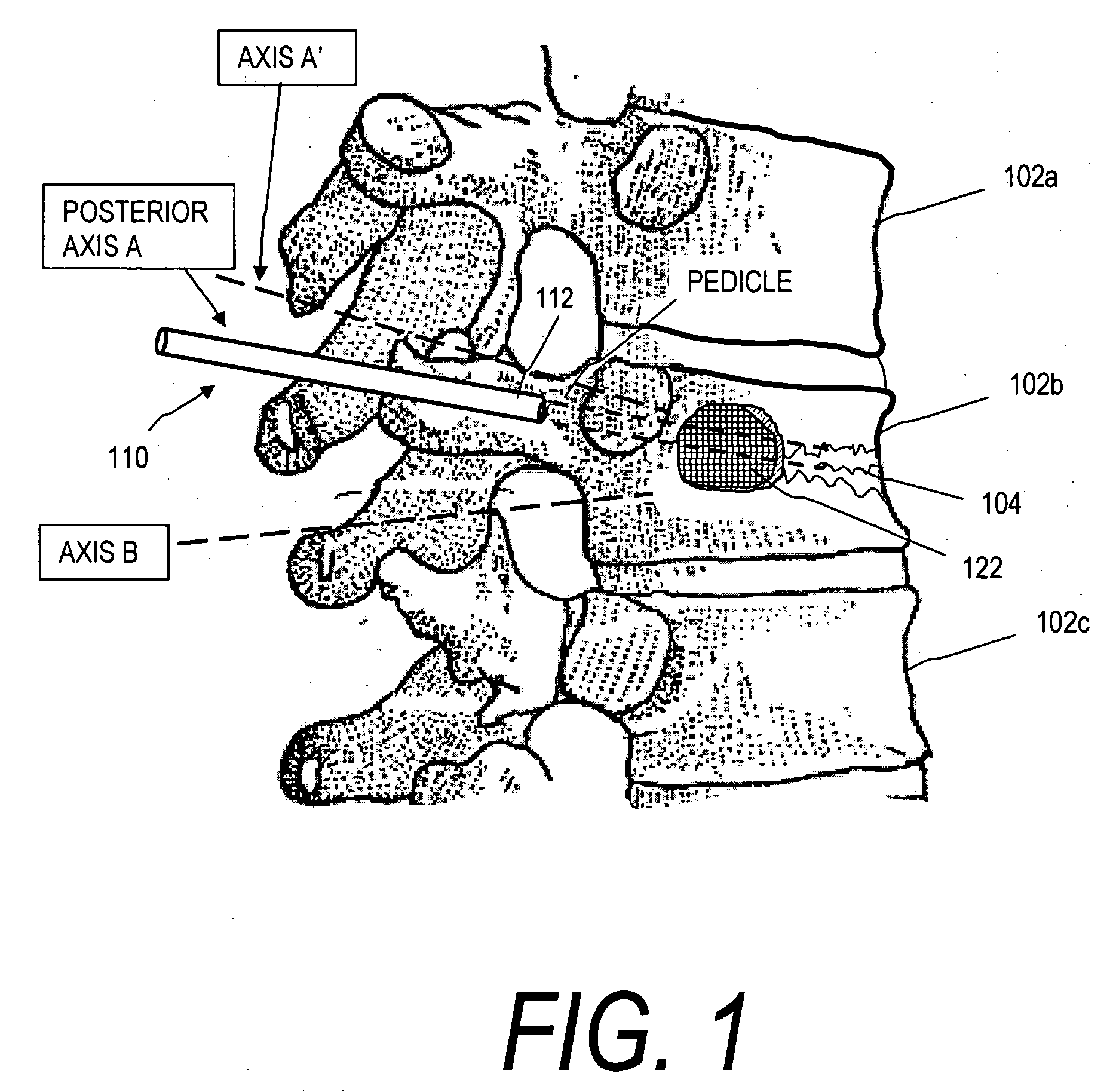
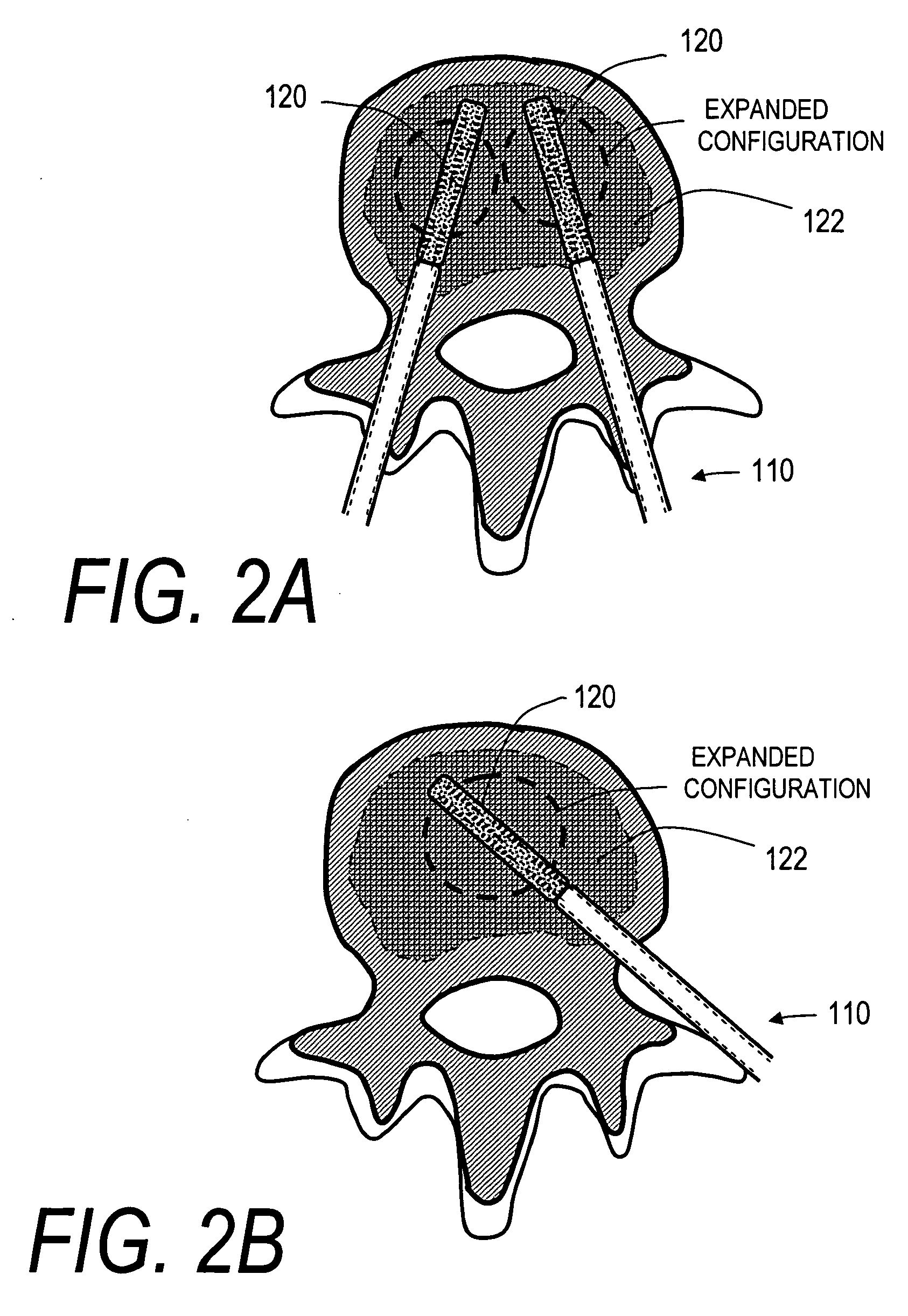
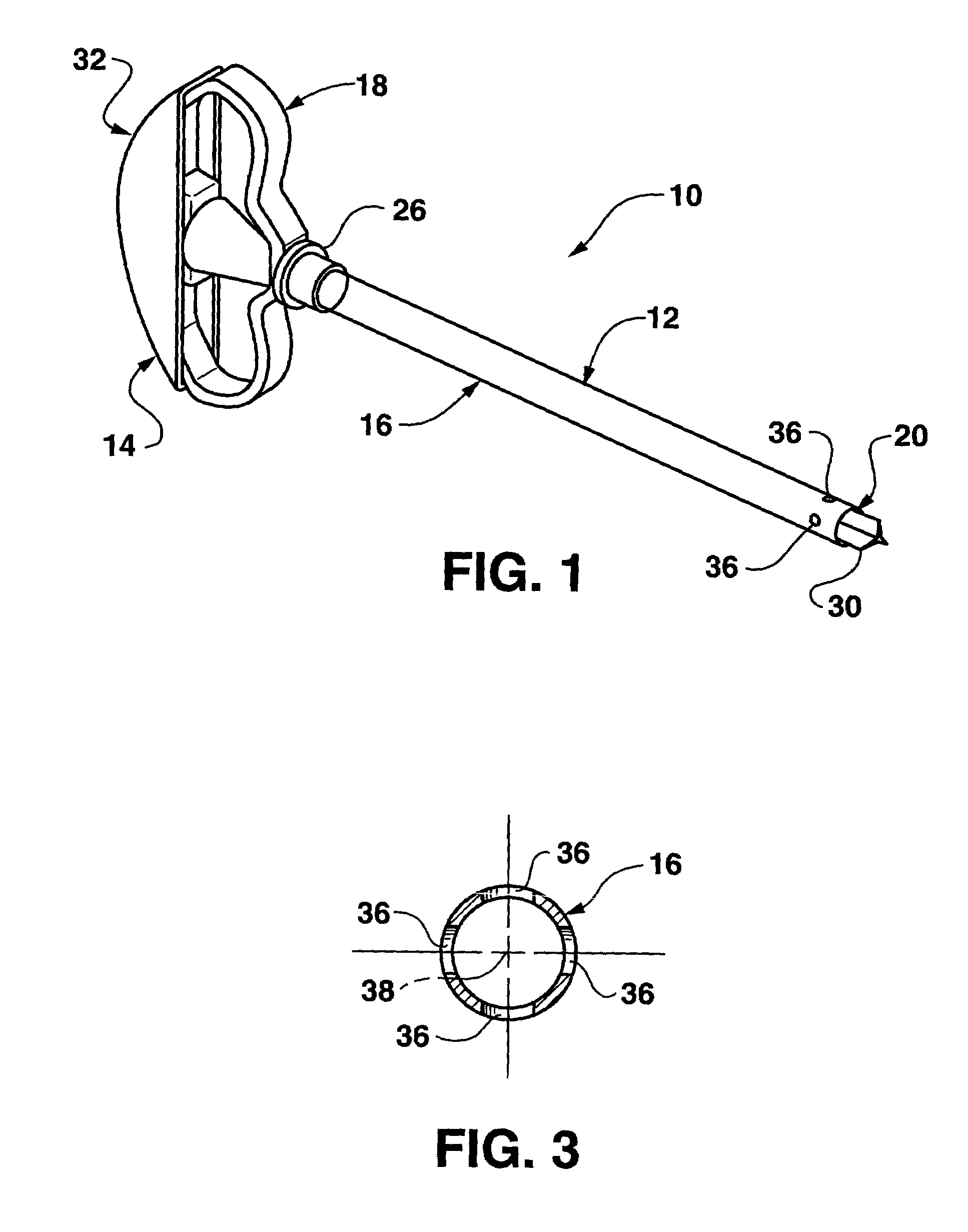
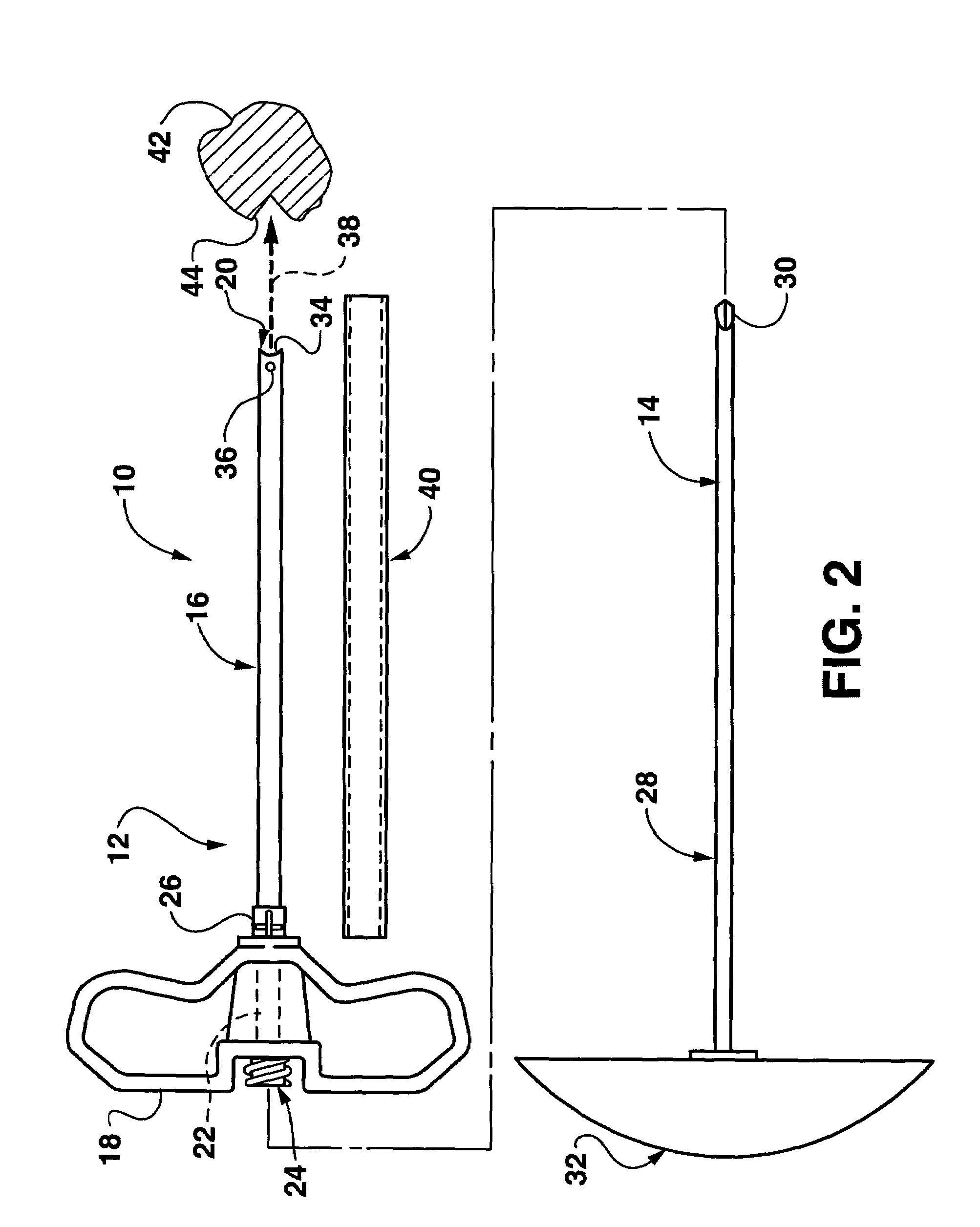
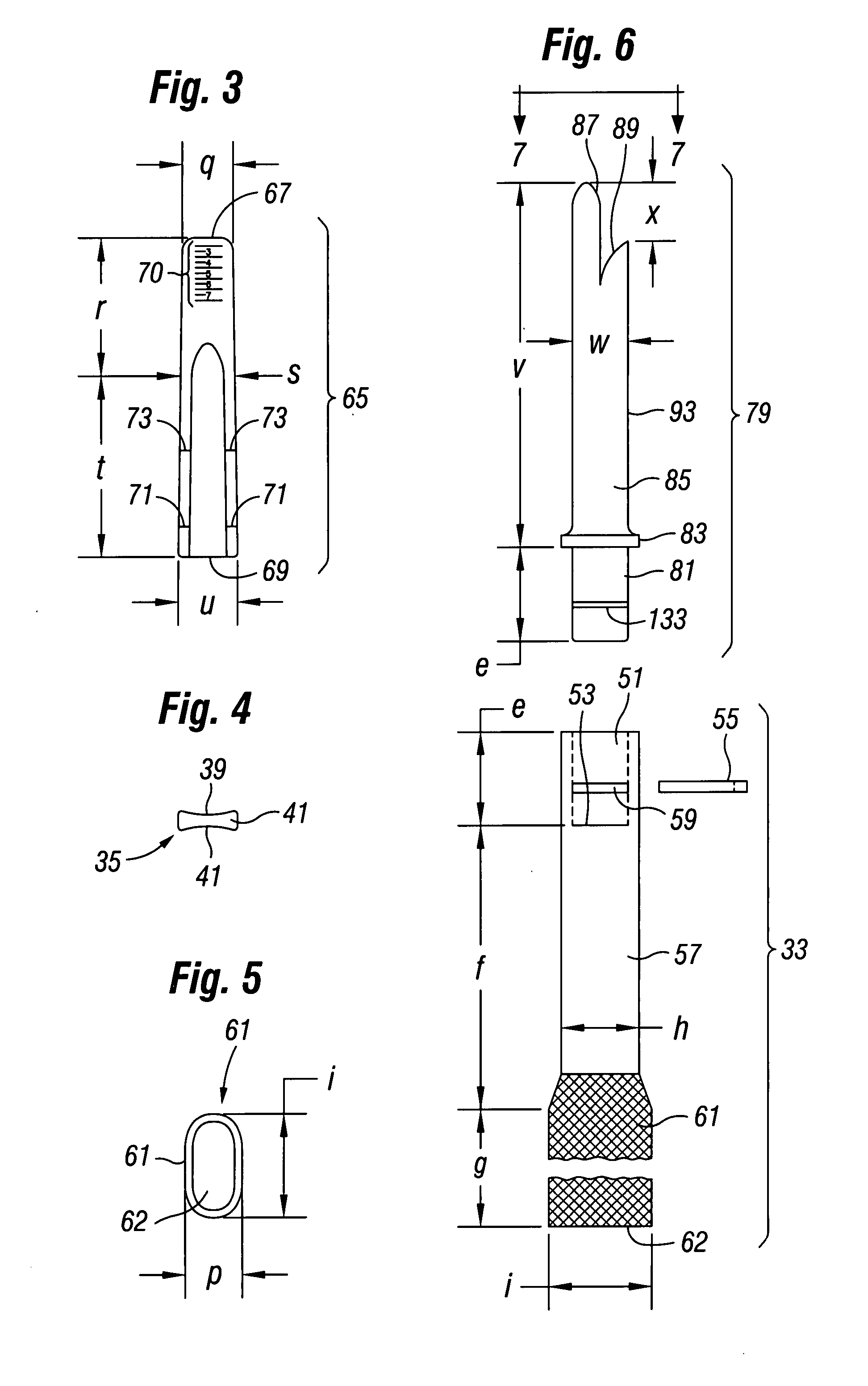
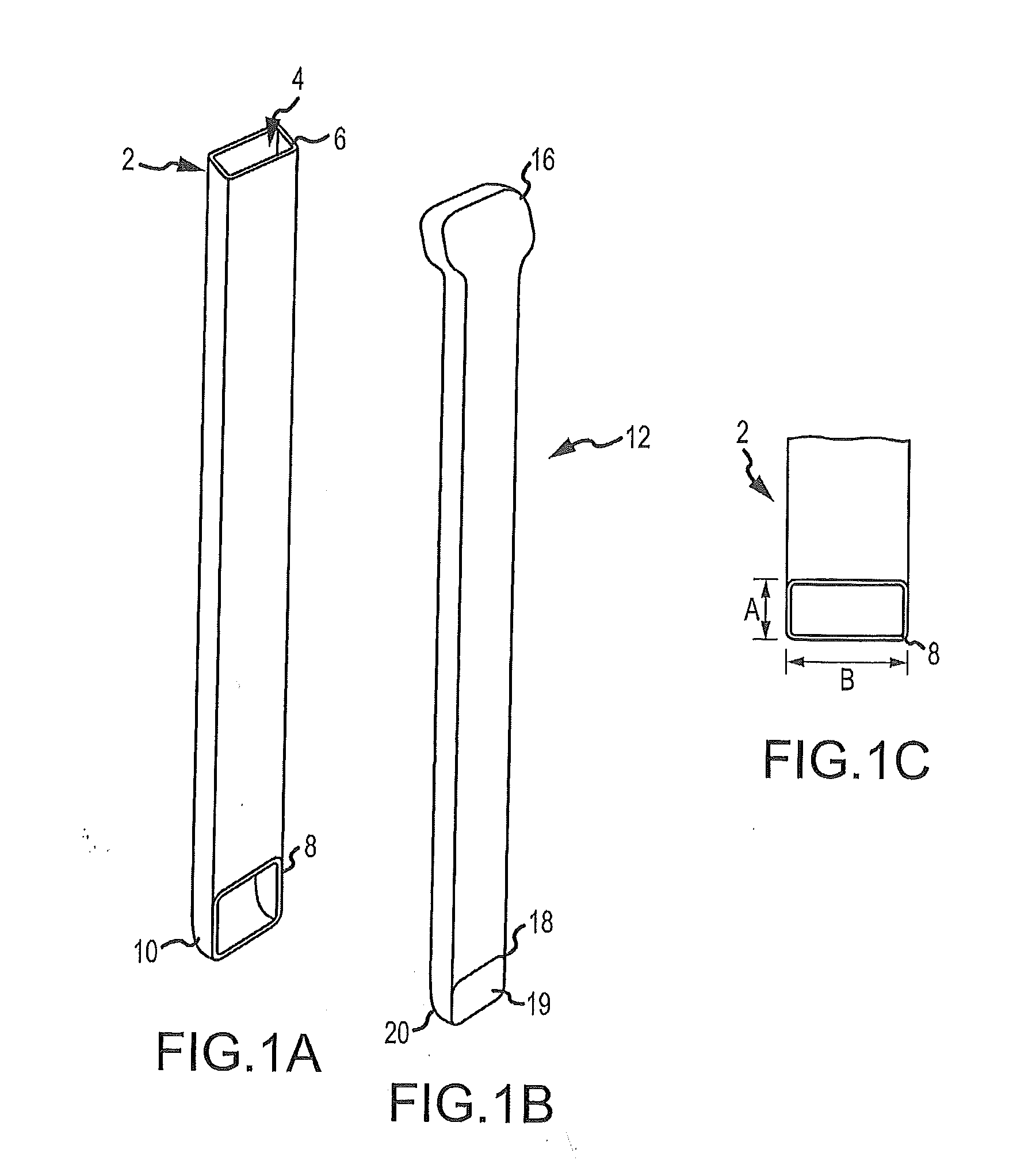
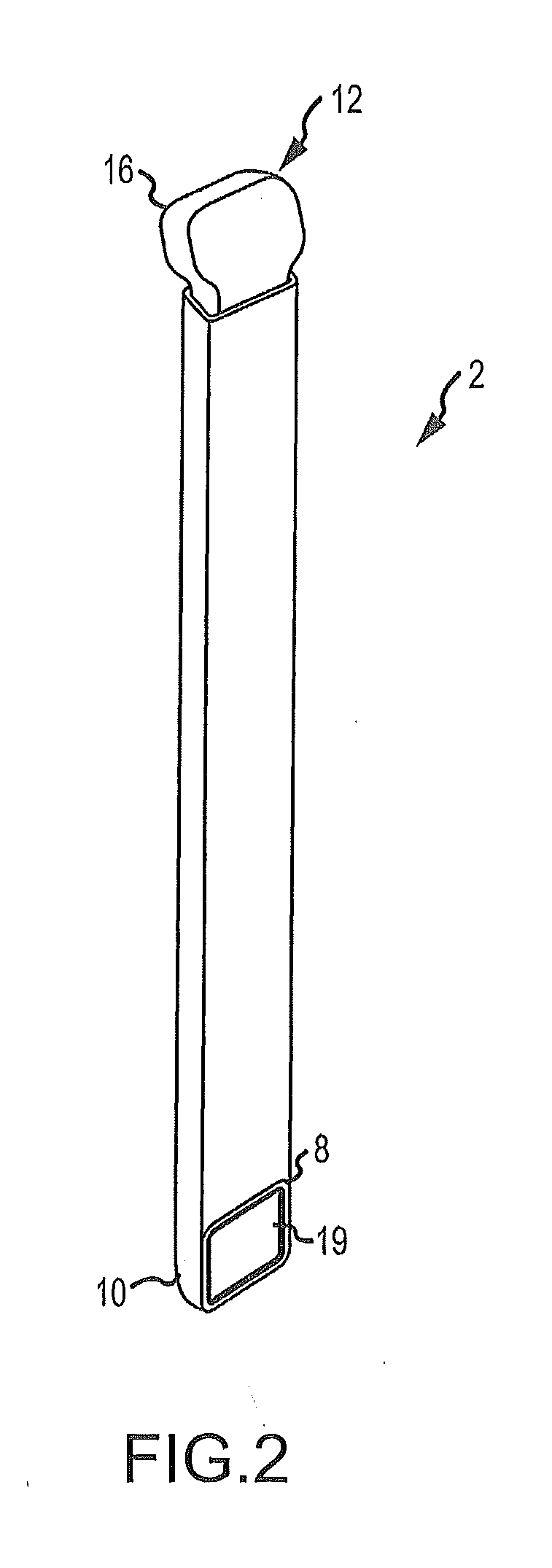
Bendable needle for delivering bone graft material and method of use
ActiveUS7066942B2Surgical needlesVaccination/ovulation diagnosticsMinimally invasive proceduresBone graft materials
A bone graft needle, particularly useful in minimally invasive procedures, is provided. The bone graft needle as well as its corresponding penetrating member may be made from bendable materials so that the combined instrument can more easily access hard to reach areas of the body.
Owner:WRIGHT MEDICAL TECH
Medical and dental implant devices for controlled drug delivery
Implantable devices and methods for use in the treatment of osteonecrosisare provided. The device includes at least one implant device body adapted for insertion into one or more channels or voids in bone tissue; a plurality of discrete reservoirs, which may preferably be microreservoirs, located in the surface of the at least one implant device body; and at least one release system disposed in one or more of the plurality of reservoirs, wherein the release system includes at least one drug selected from the group consisting of bone growth promoters, angiogenesis promoters, analgesics, anesthetics, antibiotics, and combinations thereof. The device body may be formed of a bone graft material, a polymer, a metal, a ceramic, or a combination thereof. The device body may be a monolithic structure, such as one having a cylindrical shape, or it may be in the form of multiple units, such as a plurality of beads.
Owner:MICROCHIPS INC
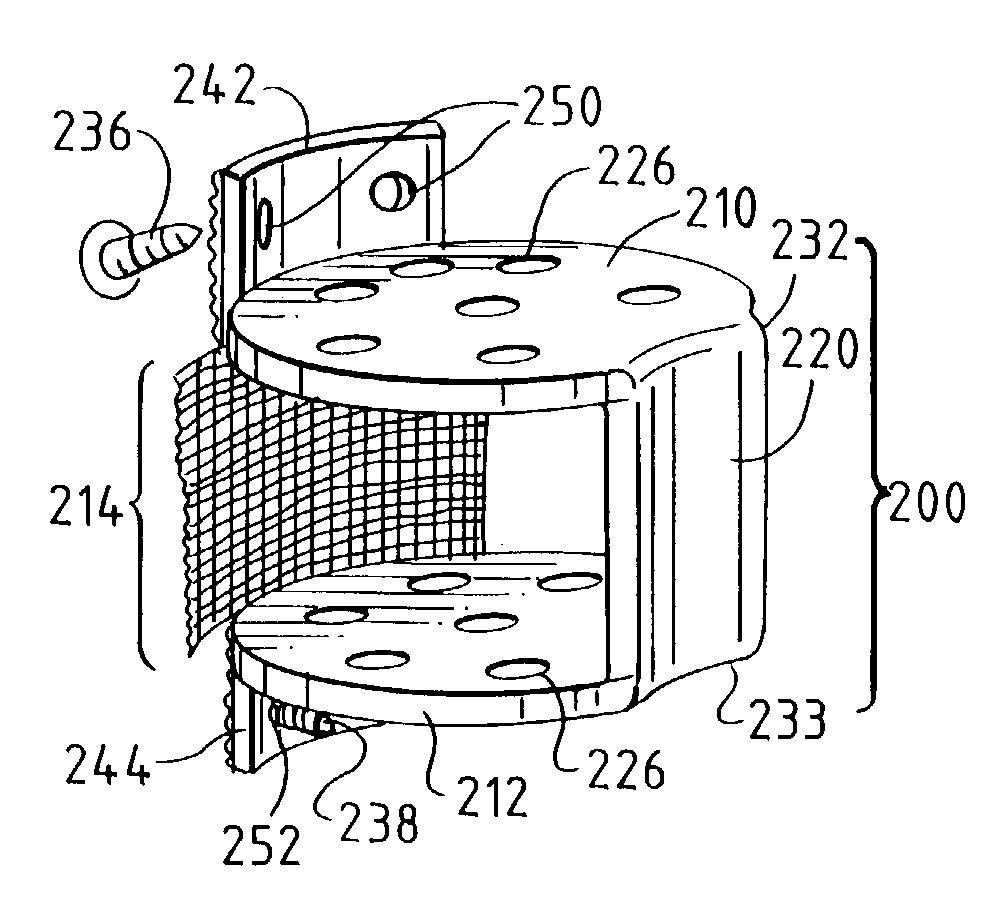
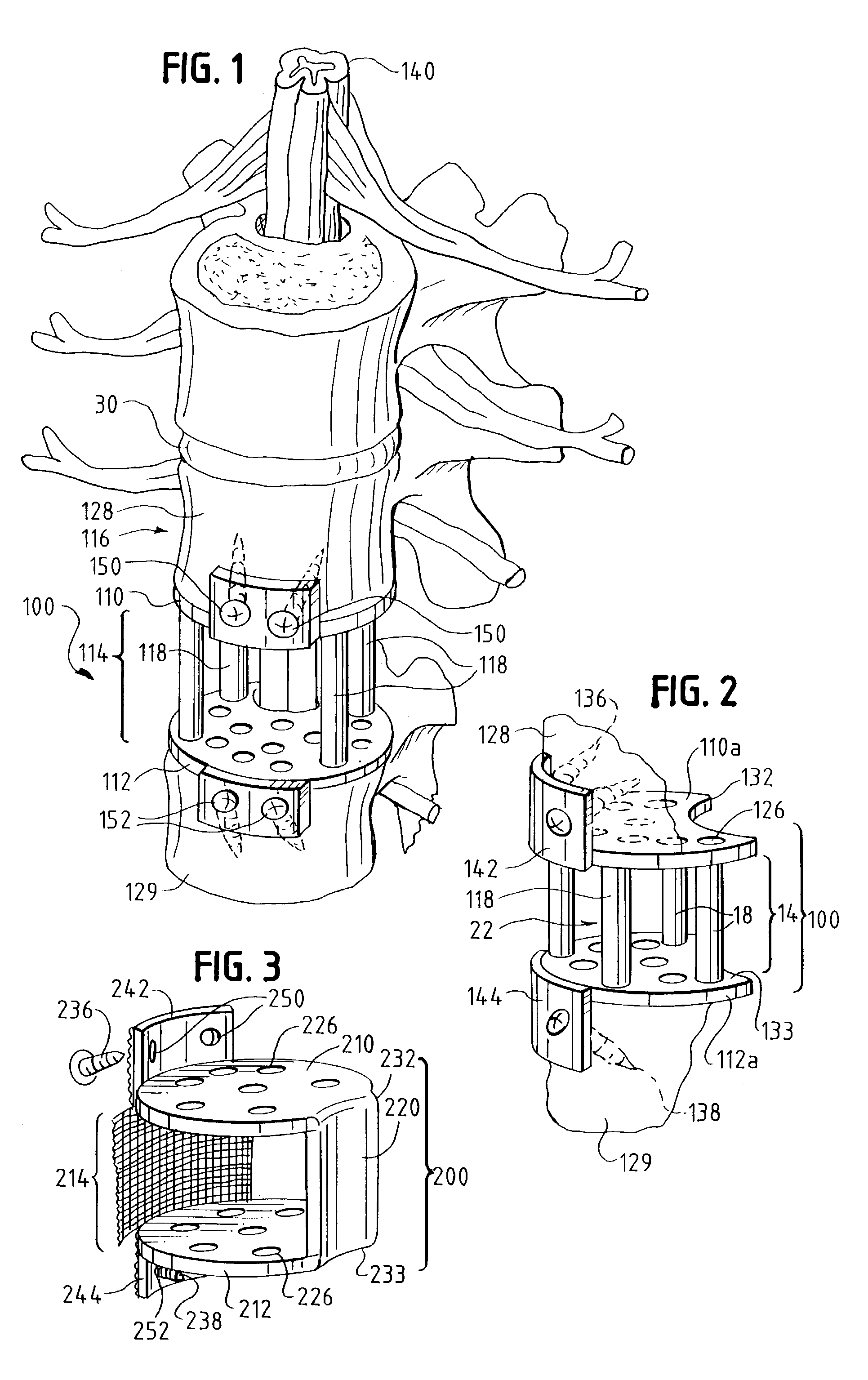
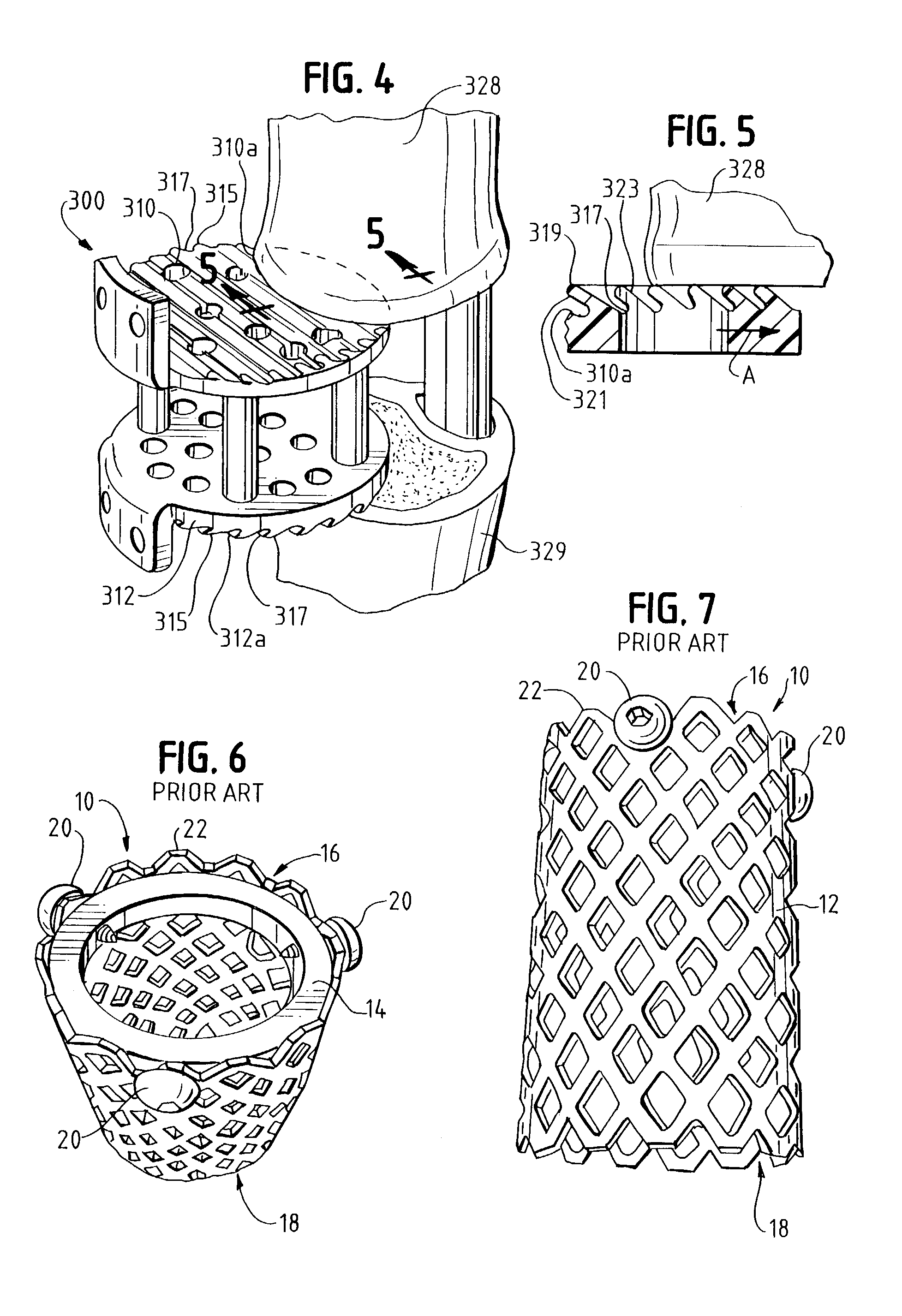
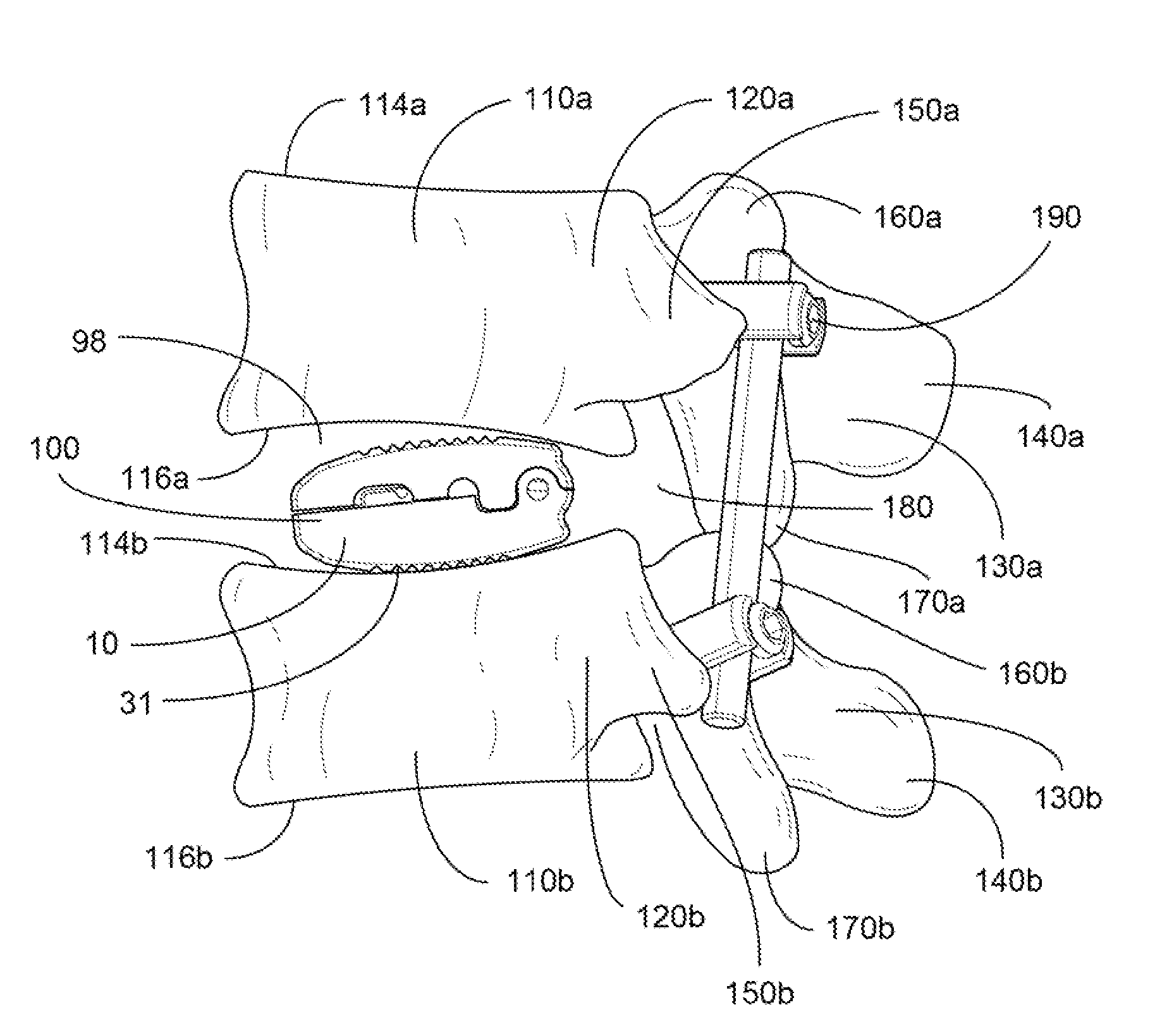
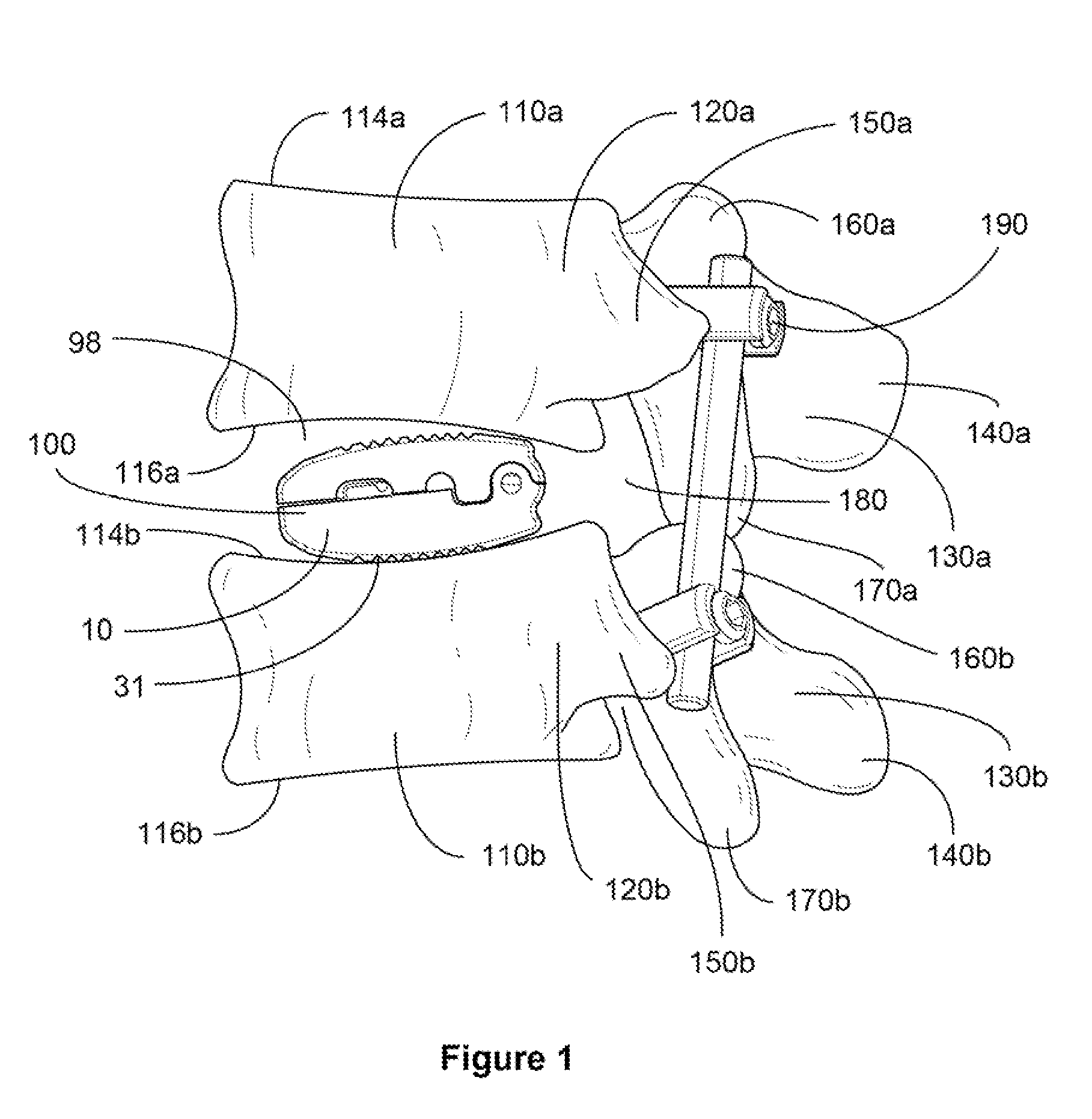
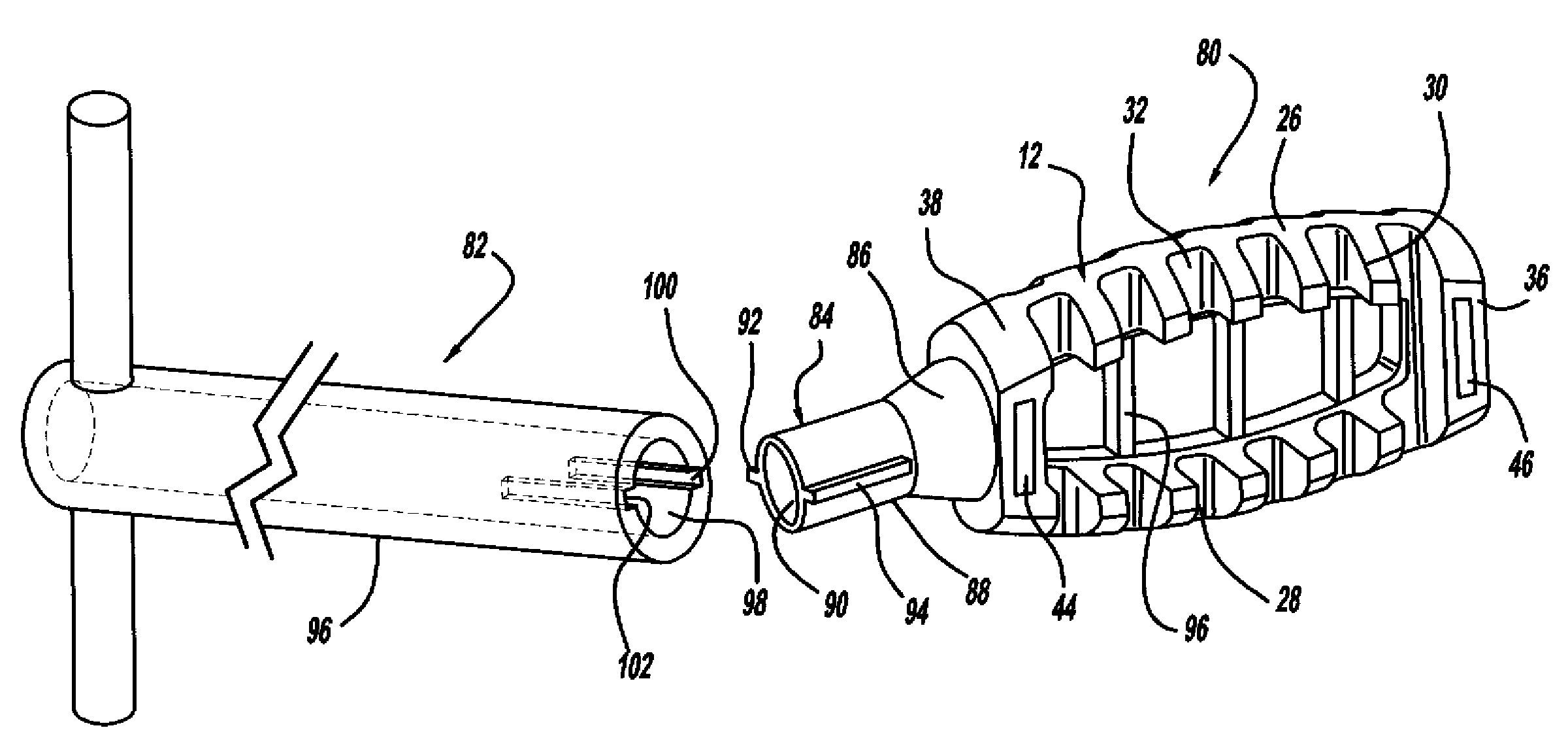
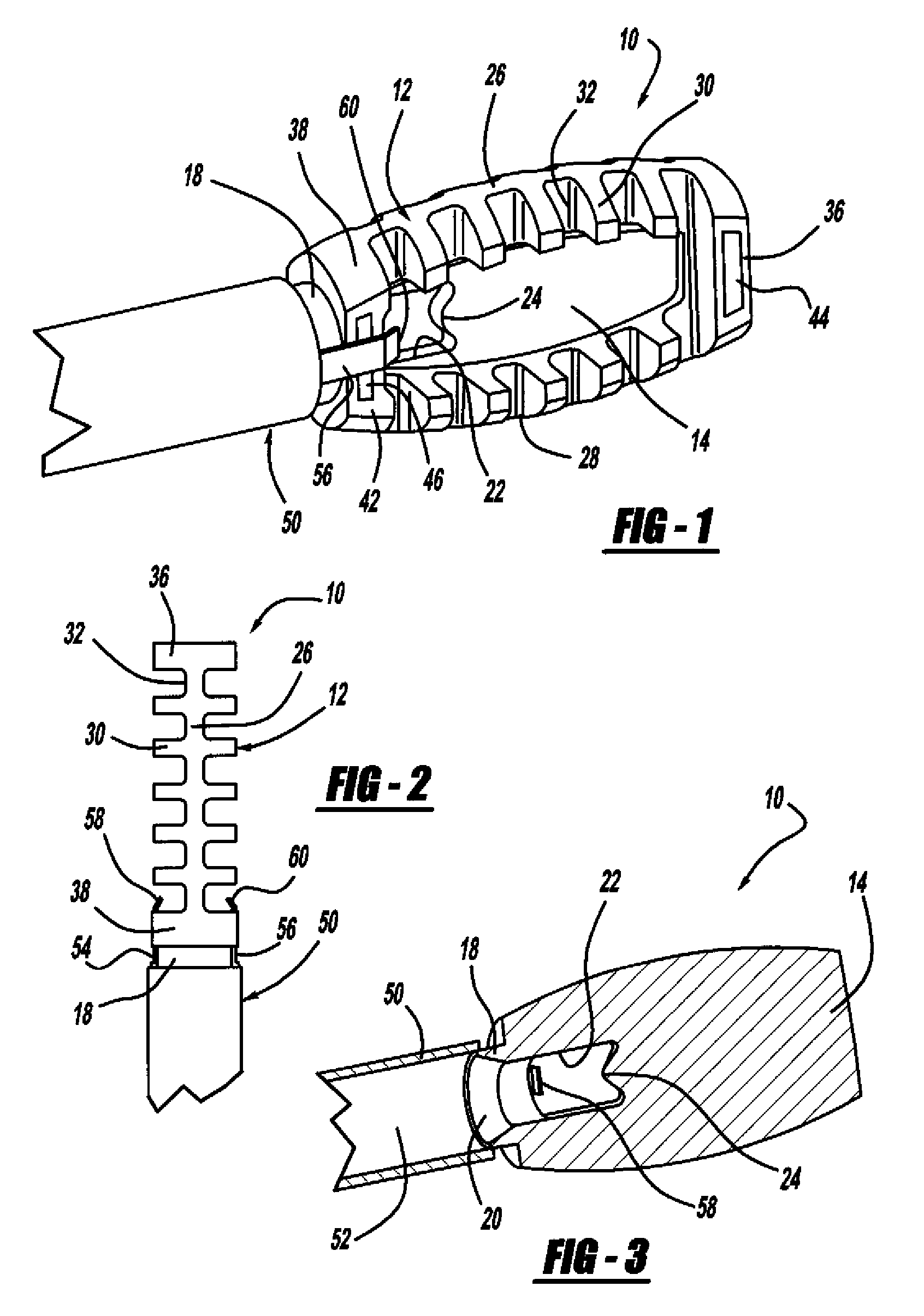
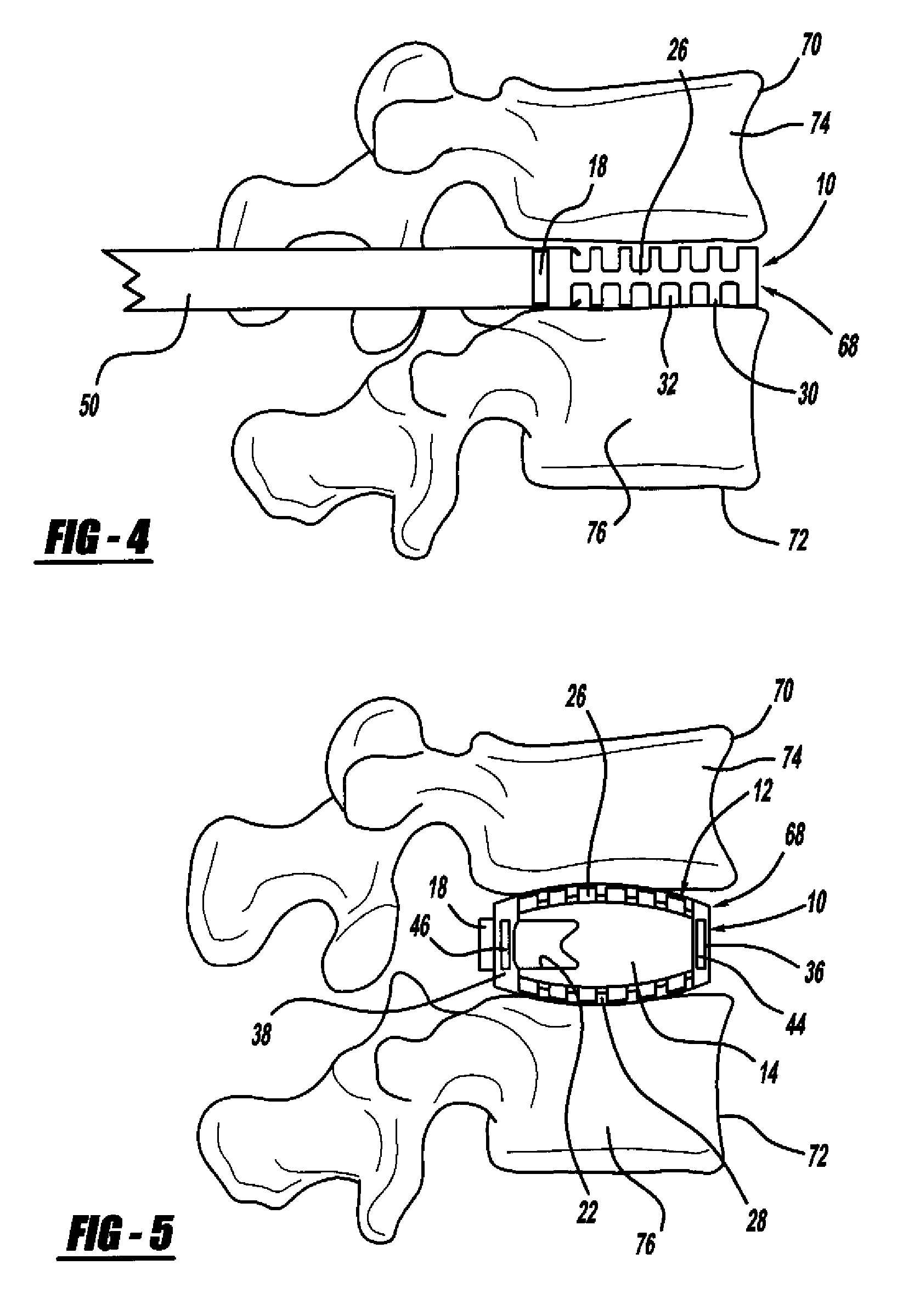
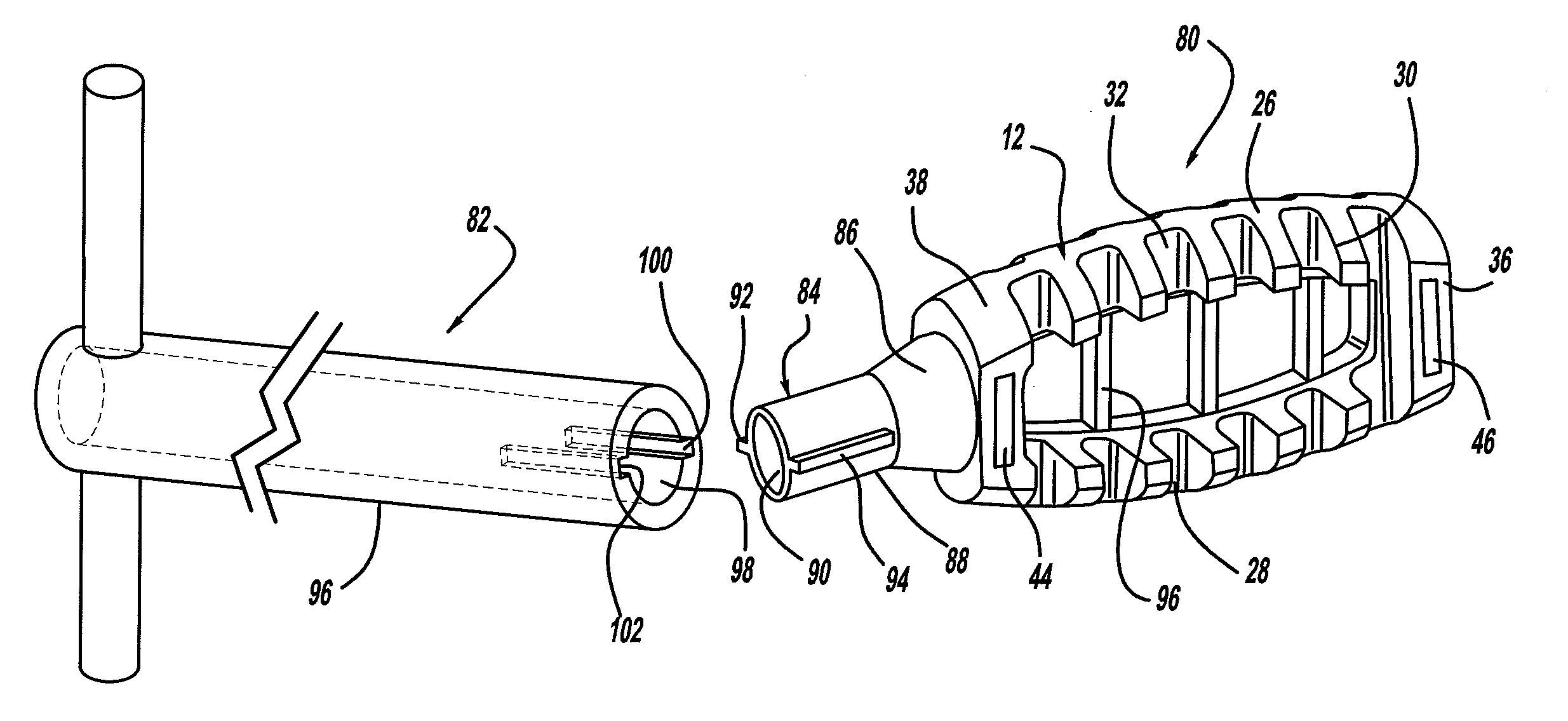
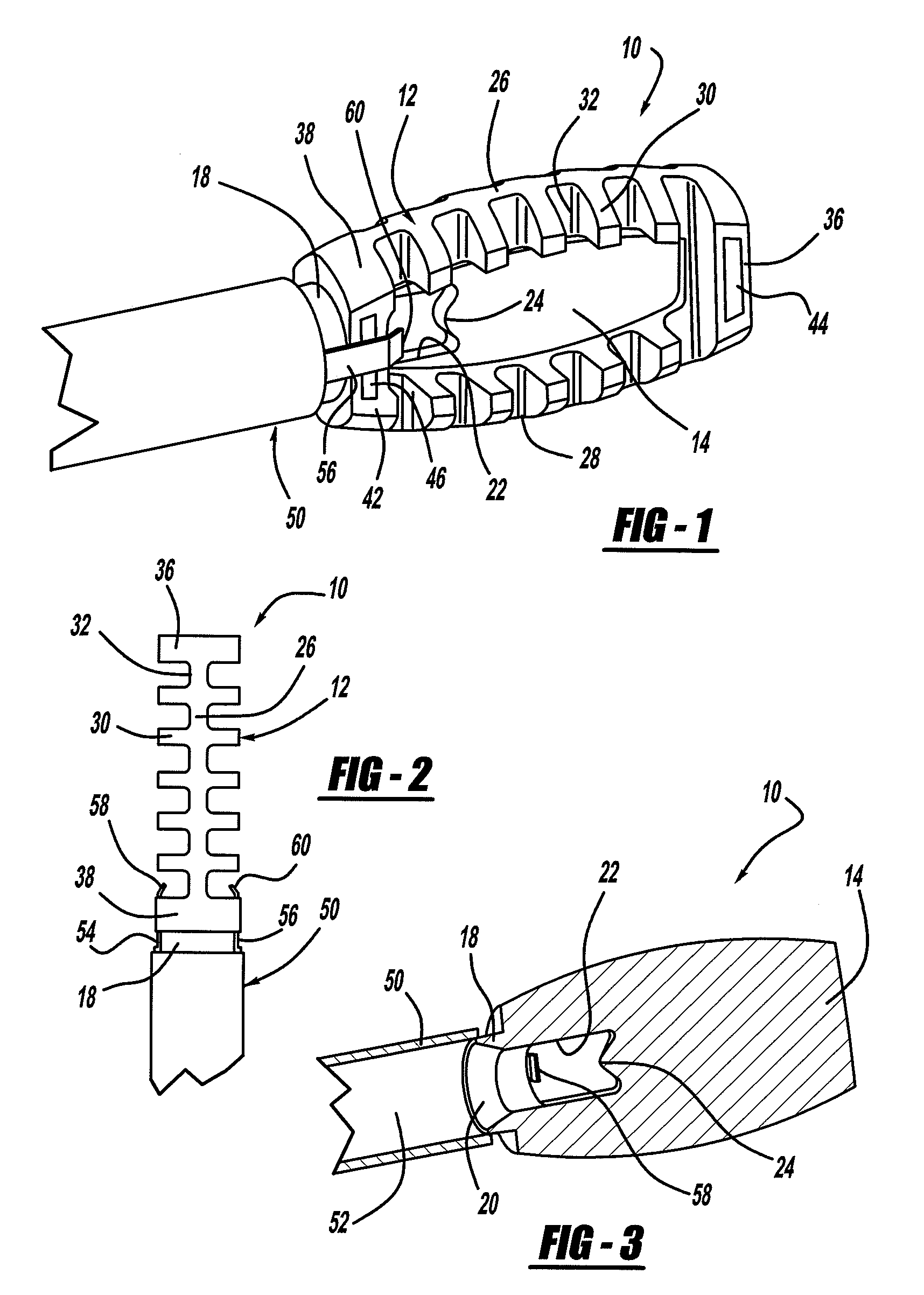
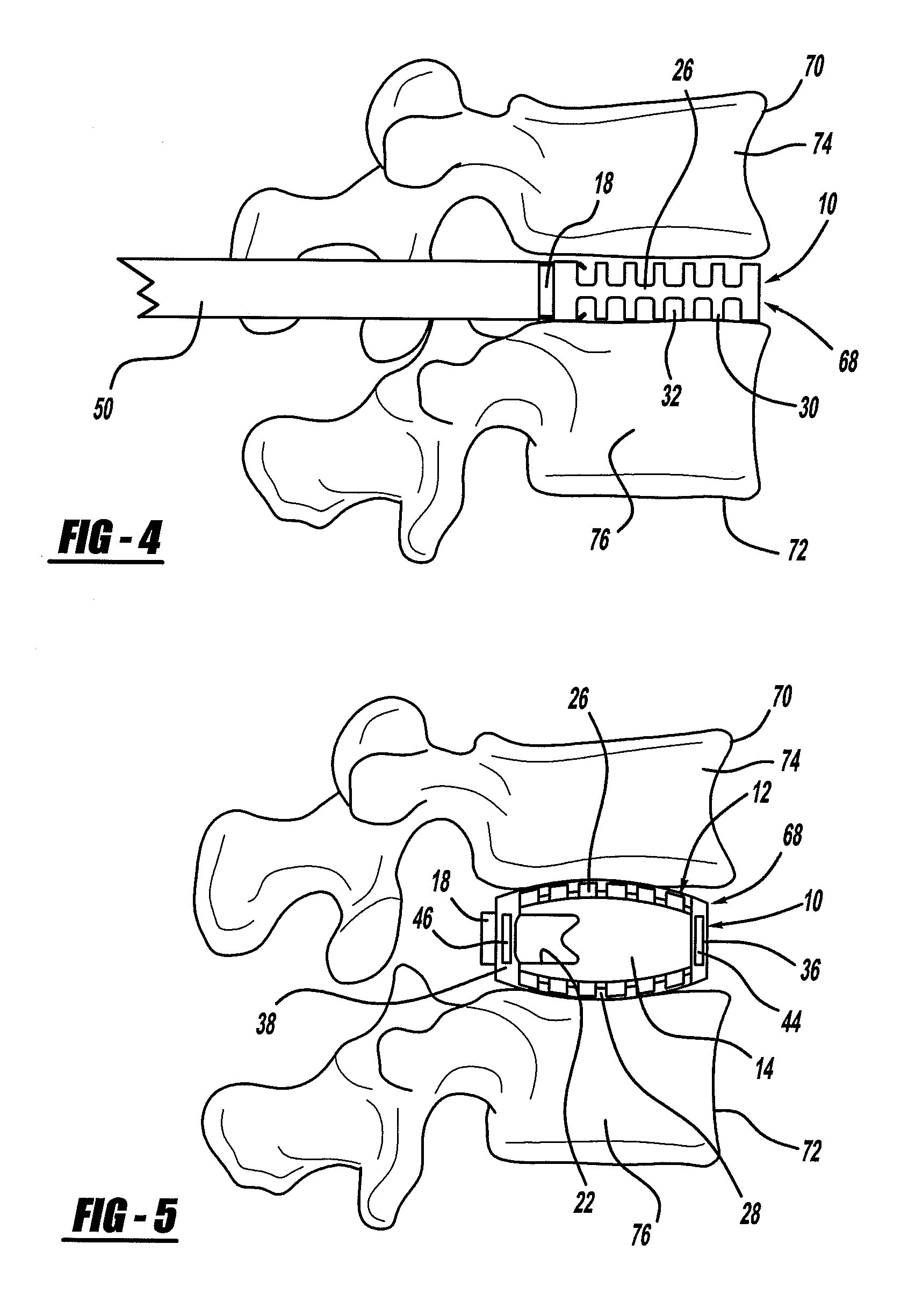
Occipitocervical plate
Methods and systems for occipital-cervical spinal fixation. A plate configured for attachment to the occipital bone has two arms extending out from either side, which turn downwards parallel to one another. A first bend is disposed in the arms, such that the arms extend down from the occipital bone behind the spinous process of the C1 and C2 vertebrae, upon installation. A second bend in the arms allows attachment to the C2 vertebra. The system may be dimensioned for pediatric installation. A bone graft material may be held in place between the cervical vertebrae and the skull by installing a cable to the installed system to retain the bone graft material in place. Methods and kits for occipital-cervical spinal fixation are also disclosed.
Owner:UNIV OF UTAH RES FOUND
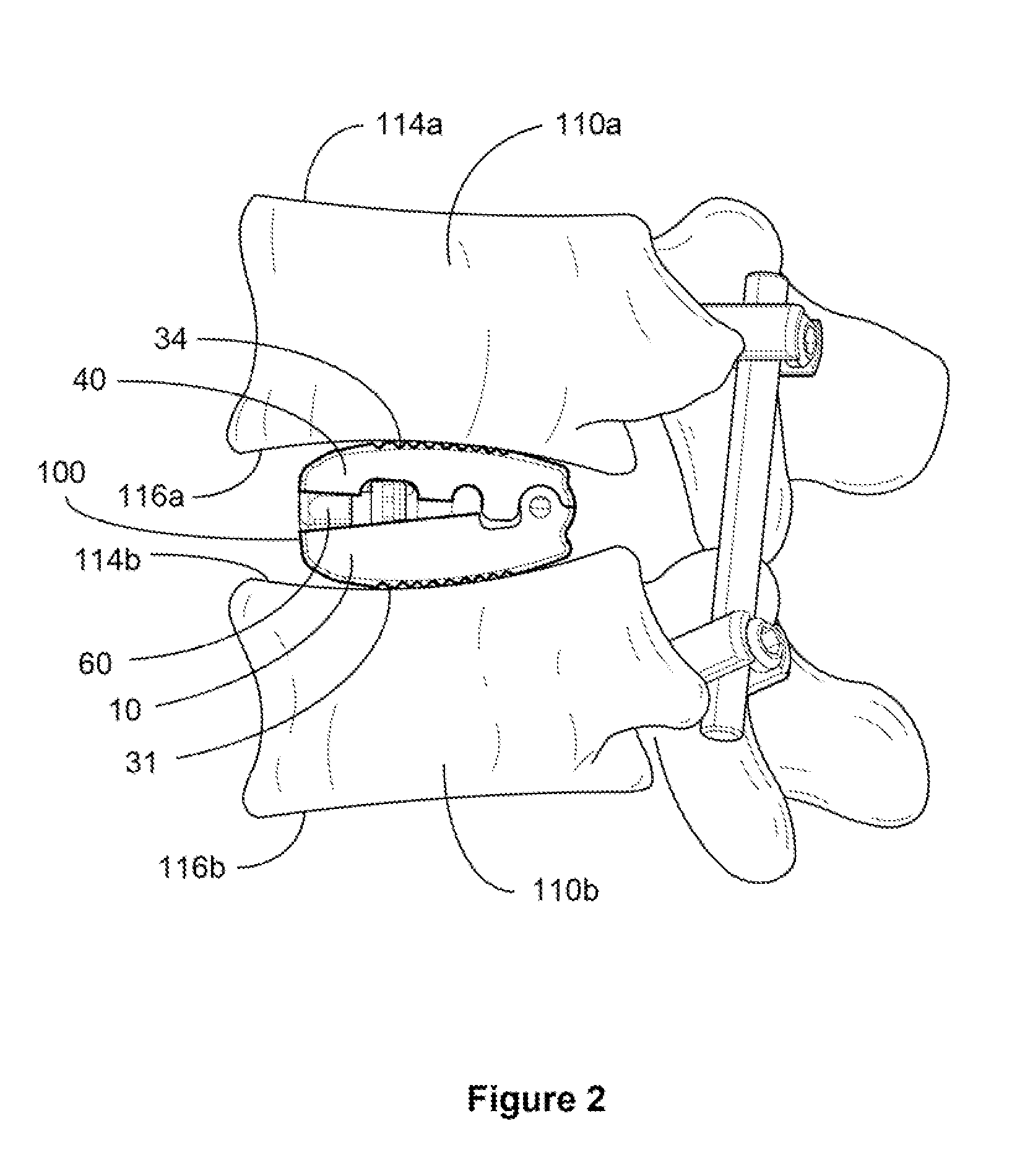
Cervical interbody device
An interbody spacer assembly includes a pair of end pieces spaced apart by a connector extending between them. The end pieces extend generally parallel to the end plates of adjoining vertebral bodies. Fasteners connect the end pieces to the vertebral bodies. Bone graft material or solid bone can be placed in the interior space defined by the end pieces and connector, which bone graft material or solid bone eventually fuses together and to the adjoining end plates through the end pieces.
Owner:WARSAW ORTHOPEDIC INC
Allograft intervertebral implant and method of manufacturing the same
ActiveUS20080082173A1Facilitating spinal fusionBone implantSpinal implantsSacroiliac jointAllograft bone
The present invention is directed to an allograft intervertebral implant sized and configured for insertion between adjacent vertebral bodies in a spinal fusion surgery. The implant is preferably manufactured from two or more pieces of allograft bone joined together by a joint, more preferably a dovetail joint. The dovetail joint being sized and configured to substantially follow the exterior shape or surface (e.g. perimeter) of the intervertebral implant. The intervertebral implant may also include one or more bone pins for joining the allograft pieces, the pins being inserted into the implant at an angle substantially perpendicular with respect to the dovetail joint. The intervertebral implant may also include one or more through-bores for receiving ostegenic or bone graft material. The intervertebral implant is preferably sized and configured for insertion during a T-PLIF or PLIF procedure.
Owner:SYNTHES USA
Spinal fusion instrumentation, implant and method
InactiveUS20080065222A1Simple methodSmall sizeBone implantSpinal implantsSpinal columnSurgical instrumentation
A system and method includes surgical instrumentation, implants, bone graft material, and measurement equipment to enable a spine fusion procedure to proceed more accurately, efficiently and safely by allowing precision measurement of the characteristics of the intervertebral space, selection of and provision of new implants, placement of an intervertebral implant, and to help overcome bone resorption all where improved healing thereafter takes place.
Owner:HAMADA JAMES S
Bone Cage with Components for Controlled Expansion
The present invention is an expandable and adjustable bone cage designed to be used in conjunction with a pedicle screw or plating fusion system. The expandable and adjustable bone cage provides structure for the placement of bone graft material between two adjacent vertebral bodies in order to stabilize or fuse the spine in a predetermined position. The expandable and adjustable bone cage is contoured for easy insertion between vertebral bodies and may be expanded after insertion to maintain, establish or increase lordosis, as well as help secure the bone cage.
Owner:RAGAB ASHRAF A +1
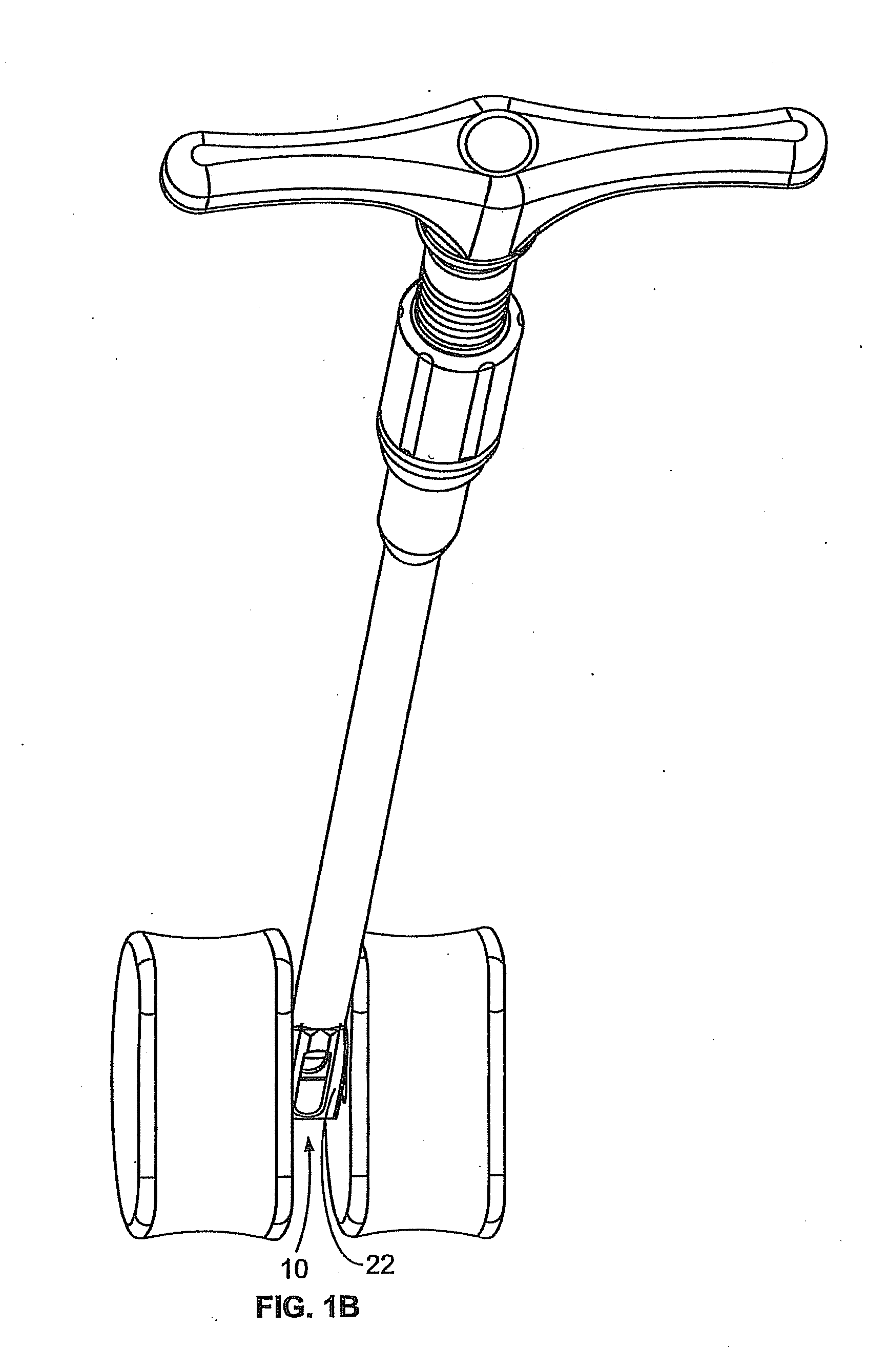
Cervical compression plate assembly
InactiveUS20050043732A1Avoid distractionsLimiting distractionInternal osteosythesisSurgical instrument detailsEngineeringCervical spine
A cervical compression plate assembly having screw receiving elements at opposite ends that are configured for engaging bone fixation screws extending from respective vertebral elements. The assembly permits the distance between the screw receiving elements at opposite ends to be shortened but prevents the distance from increasing. A spring mechanism is housed within the plate assembly and is configured for continuously urging the screw receiving elements at opposite ends together for thereby providing continuous compressive loading on bone graft material disposed between the vertebral elements.
Owner:DALTON BRIAN E
System and method for spinal fixation
InactiveUS20100331891A1Increase ratingsQuality of the joint formed between fixated bones can be improvedSuture equipmentsInternal osteosythesisSpinal columnDilator
A system and method of bone fixation are provided for improving the bone growth and stability of the fixated bones. For example, a target site for a bone fixation procedure can be accessed at a facet of a first vertebra using a tissue dilator. Bone material can be disrupting from or at the target site, and a bone fixation device can be installed to fix the first vertebra relative to a second vertebra. The disruption and / or removal of the bone material, such as by rasping facets or a facet joint of the first vertebra and the second vertebra, can tend to promote bone growth. Further, it is contemplated that bone graft material can be inserted at the target site, such as into a joint space formed between facets of the first vertebra and the second vertebra.
Owner:INTERVENTIONAL SPINE
Balloon technologies for tissue repair
A medical device containing an inflatable balloon structure for use in minimally invasive surgery and minimally invasive diagnostic and therapeutic procedures are described herein. The device is delivered by a catheter and expanded using gases, liquids or liquids that solidify in situ. The inflatable balloon may be constructed from a wide variety of materials and may be reinforced by supporting structures, when necessary. The device may form an endoprosthesis in a patient. In the preferred embodiment, the device is used in spinal fusion. Optionally, the device may also be used in combination with bone graft materials and bioactive factors.
Owner:KUROS BIOSURGERY AG
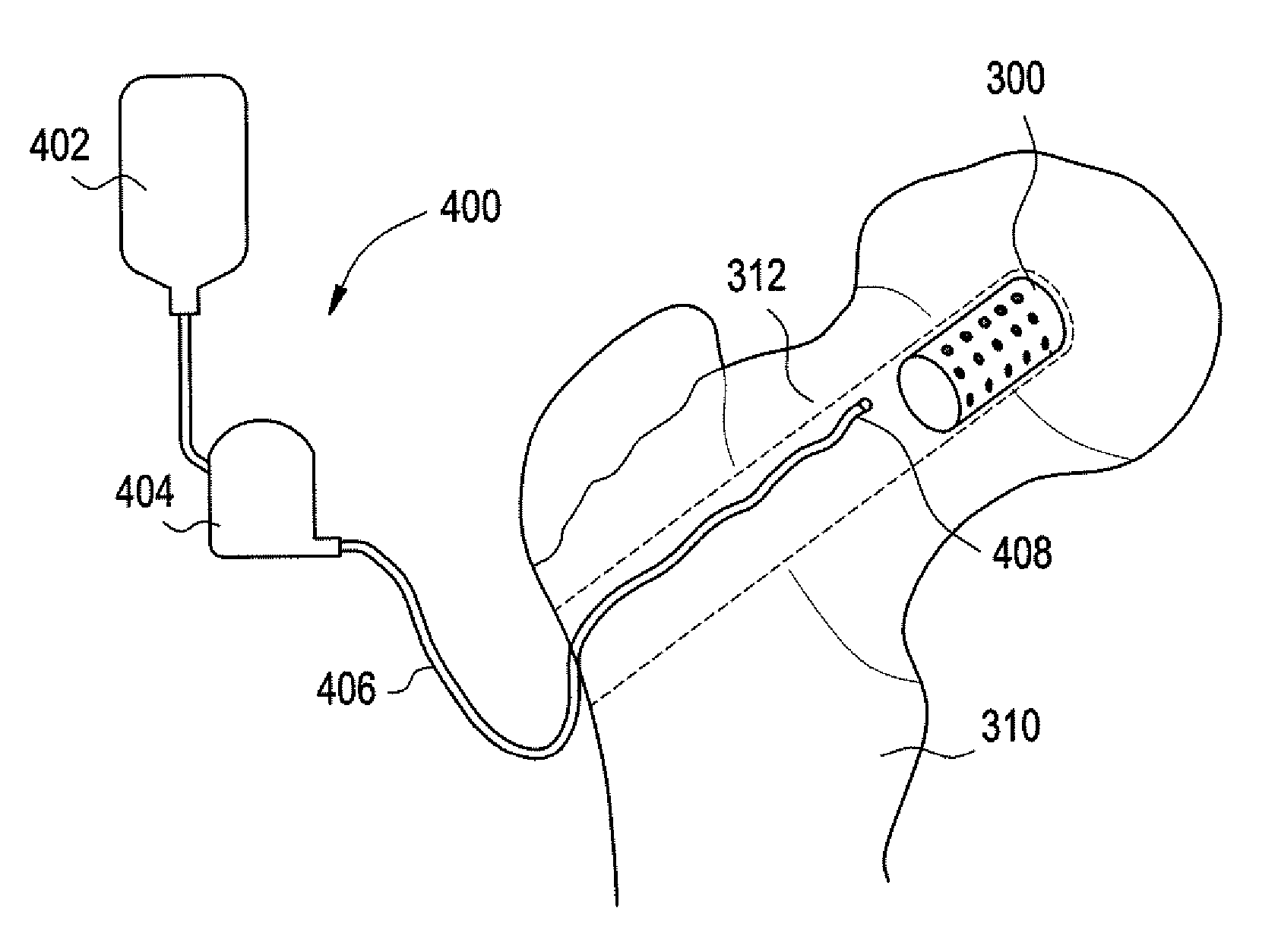
Intervertebral spacer
InactiveUS20100262245A1Convenient introductionEasy to installBone implantSpinal implantsIntervertebral spaceBone grafting
Disclosed is a delivery system for an intervertebral spacer and a bone grafting material in the form of a unitary device which comprises a spacer disengagingly attached to a hollow handle. The handle comprises a chamber and bone grafting material-advancing means within the chamber for introducing the bone grafting material from the chamber into and around the spacer and the intervertebral spaces. The handle is disengageable from the spacer after serving its purpose of facilitating placement of the spacer and the discharge of the bone grafting material into the spacer and the intervertebral space.The intervertebral spacer implant portion is attached to the handle portion at the exit port of the chamber, the point of attachment constituting the entry port of the intervertebral spacer implant portion.The spacer has voids therein capable of receiving the bone grafting material from the chamber, and the chamber, the spacer implant portion voids, and the intervertebral spaces are in flow communication with each other whereby bone graft material expressed from the chamber is capable of entering the intervertebral spacer implant voids and the intervertebral spaces.
Owner:ALFARO ARTHUR A +1
Interbody spinal fusion device
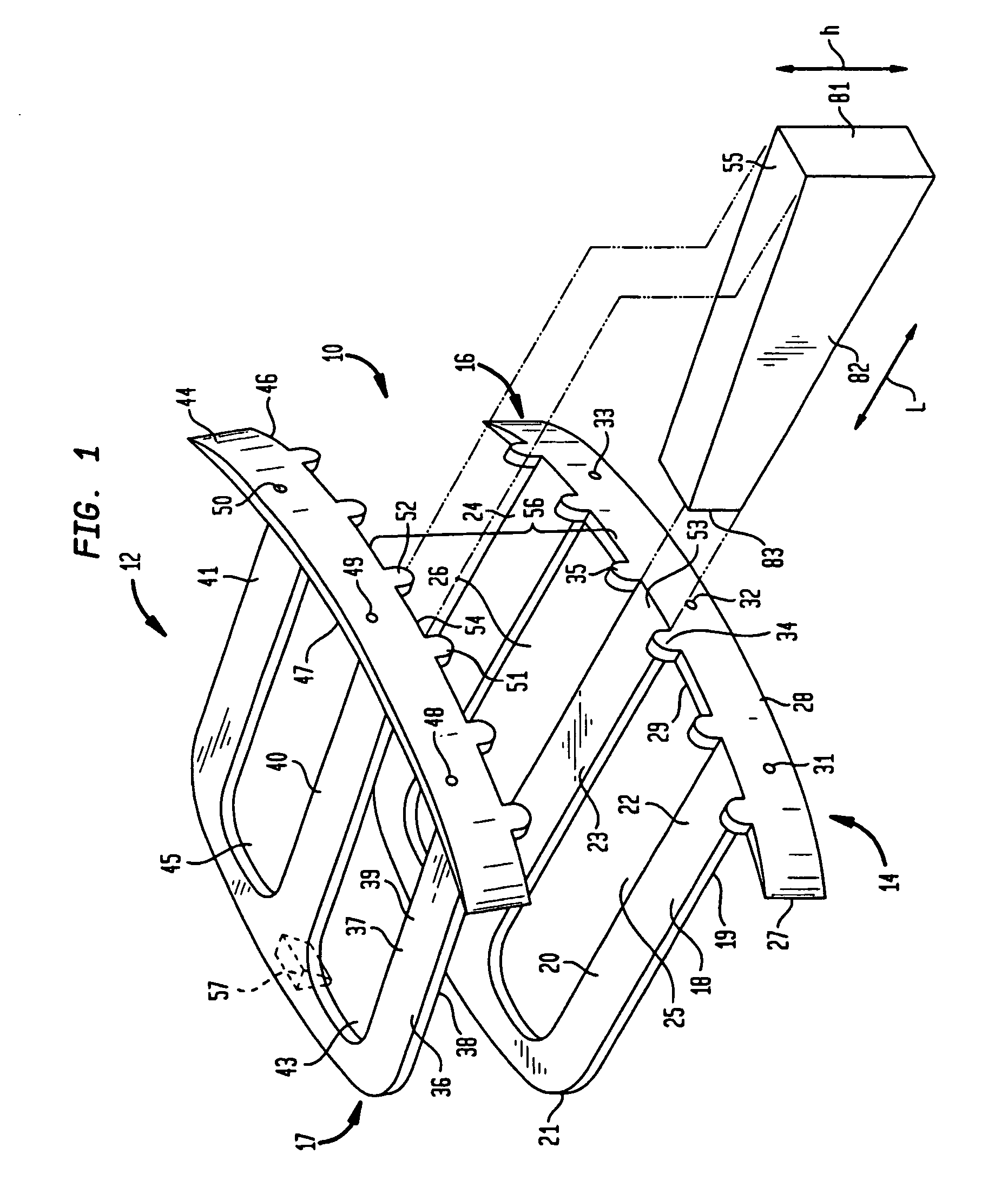
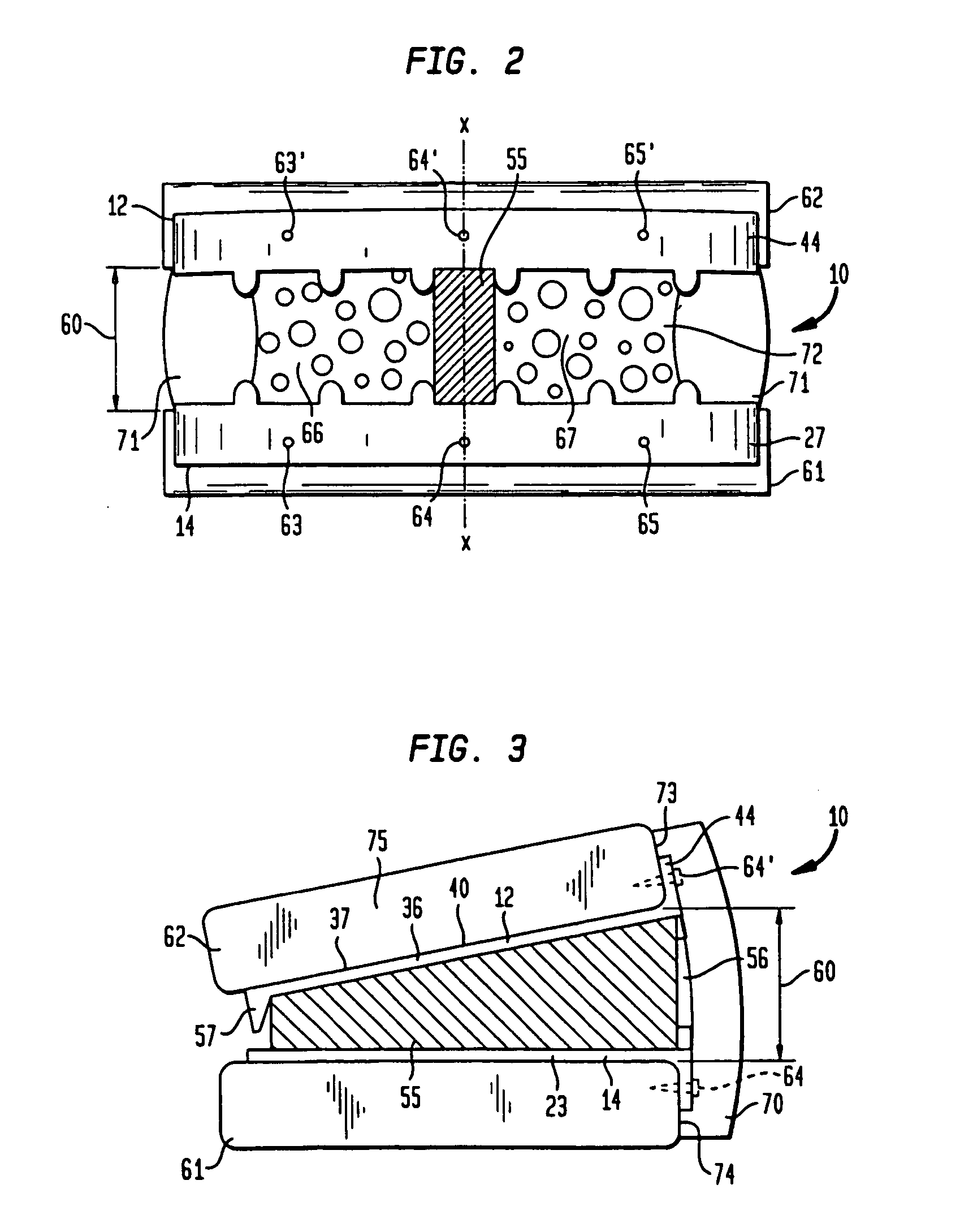
InactiveUS20070276375A1Promote bone fusionEasy maintenanceInternal osteosythesisBone implantLamina terminalisAnterior surface
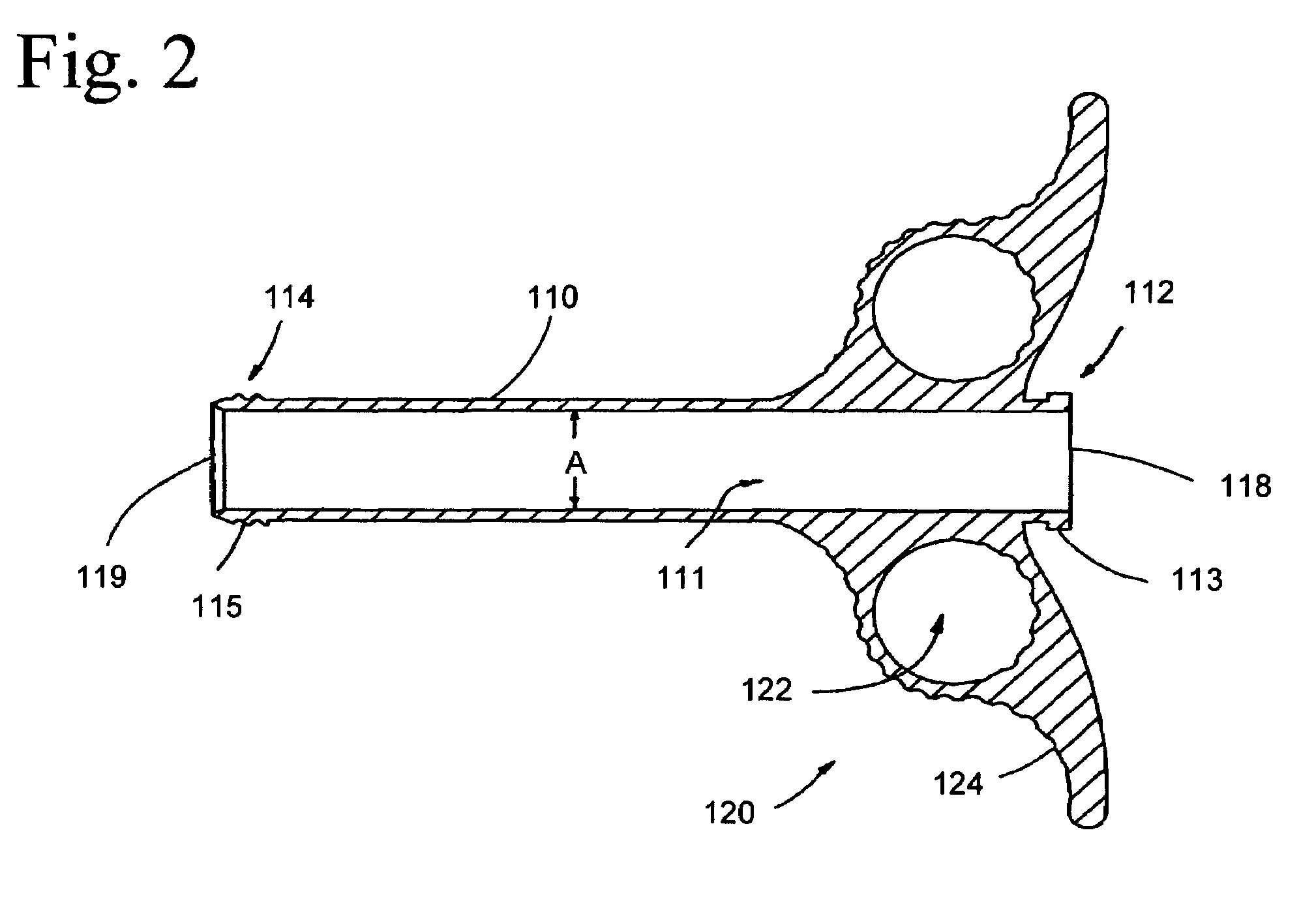
A pair of flat support plates in a spinal fusion implant device contact and rest against the softer, central cancellous bone portion of respective endplates of adjacent vertebrae. Each support plate has a front template that is orthogonal to the plate and is bent to communicate with the anterior surface of the hard cortical endplate the vertebrae. A wedge-shaped support strut in a central channel between the two plates is configured to vary the distance between the support plates such that the height of the device proximate the anterior end is greater than the height of the device at the posterior end to maintain the natural lordosis of the spine. Channels formed on either side of the support strut are filled with bone graft material and contact the endplates of the vertebrae through large openings in the flat support plates to facilitate fusion.
Owner:RAPP LAWRENCE G
Bioactive bone graft substitute
The invention relates to biocompatible bone graft materials for repairing bone defects and the application of such bone graft materials.
Owner:ORTHOVITA INC
Bone cage with components for controlled expansion
The present invention is an expandable and adjustable bone cage designed to be used in conjunction with a pedicle screw or plating fusion system. The expandable and adjustable bone cage provides structure for the placement of bone graft material between two adjacent vertebral bodies in order to stabilize or fuse the spine in a predetermined position. The expandable and adjustable bone cage is contoured for easy insertion between vertebral bodies and may be expanded after insertion to maintain, establish or increase lordosis, as well as help secure the bone cage.
Owner:RAGAB ASHRAF A +1
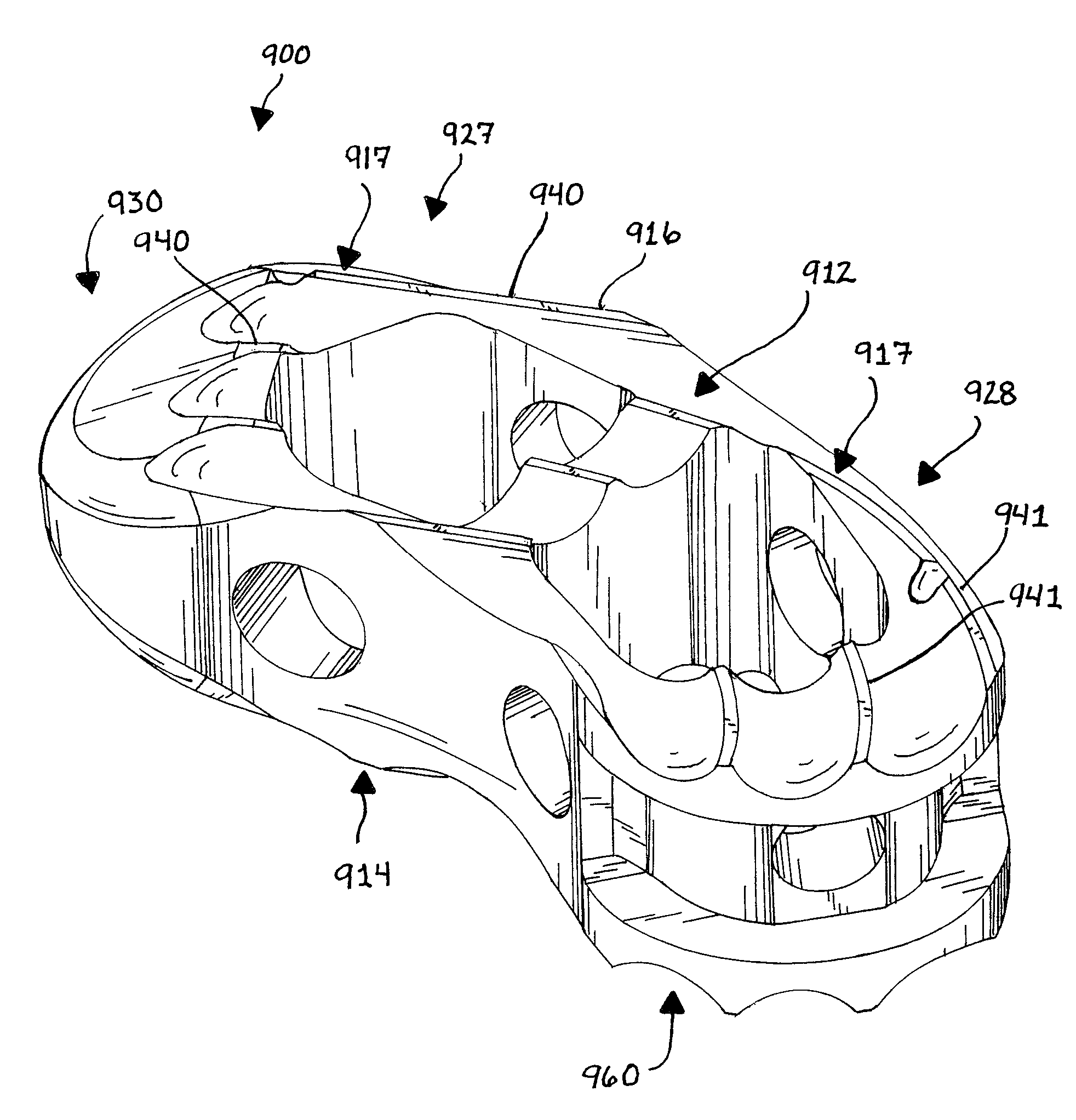
Expandable interbody implant
ActiveUS20160120660A1Improved driving linkSmoothly and reliably expandInternal osteosythesisSpinal implantsIntervertebral discBone growth
An expandable interbody implant adapted for insertion at least into a disc space, between two adjacent vertebrae of a spine. An upper member and a lower member are pivotally connected at least by a drive link. A bone graft storage portion packed with a selected volume of bone growth material. A translational wall is provided between the bone graft storage portion and a trailing end of the implant. An actuator engages the bone graft storage portion, pushing it forward into engagement with the drive link, which pivots upward, moving the upper member upward away from the lower member. Upward movement of the upper member raises the translational wall to a position to assist in retention of the selected volume bone graft material within the hollow portion of the bone graft storage portion. Upward rotation of the drive link brings a face thereof into contact with a front of the bone graft storage portion, thereby retaining near constant volume of the bone graft material.
Owner:WARSAW ORTHOPEDIC INC
Biological delivery system with adaptable fusion cage interface
ActiveUS20160106551A1Control volumeIncreased traumaBone implantJoint implantsSurgical siteBiological materials
The present invention relates to an apparatus and method for near-simultaneous and integrated delivery of bone graft material during the placement of surgical cages or other medical implants in a patient's spine. The integrated fusion cage and graft delivery device according to various embodiments delivers and disperses biologic material through a fusion cage to a disc space and, without withdrawal from the surgical site, may selectably detach the fusion cage for deposit to the same disc space. The integrated fusion cage and graft delivery device is formed such that a hollow tube and plunger selectively and controllably place bone graft material and a fusion cage in or adjacent to the bone graft receiving area. In one embodiment, the integrated fusion cage is an expandable integrated fusion cage.
Owner:SPINAL SURGICAL STRATEGIES INC
Minimally Invasive Interbody Device Assembly
InactiveUS20080172128A1Restore heightEasy to placeInternal osteosythesisJoint implantsIntervertebral diskInstrumentation
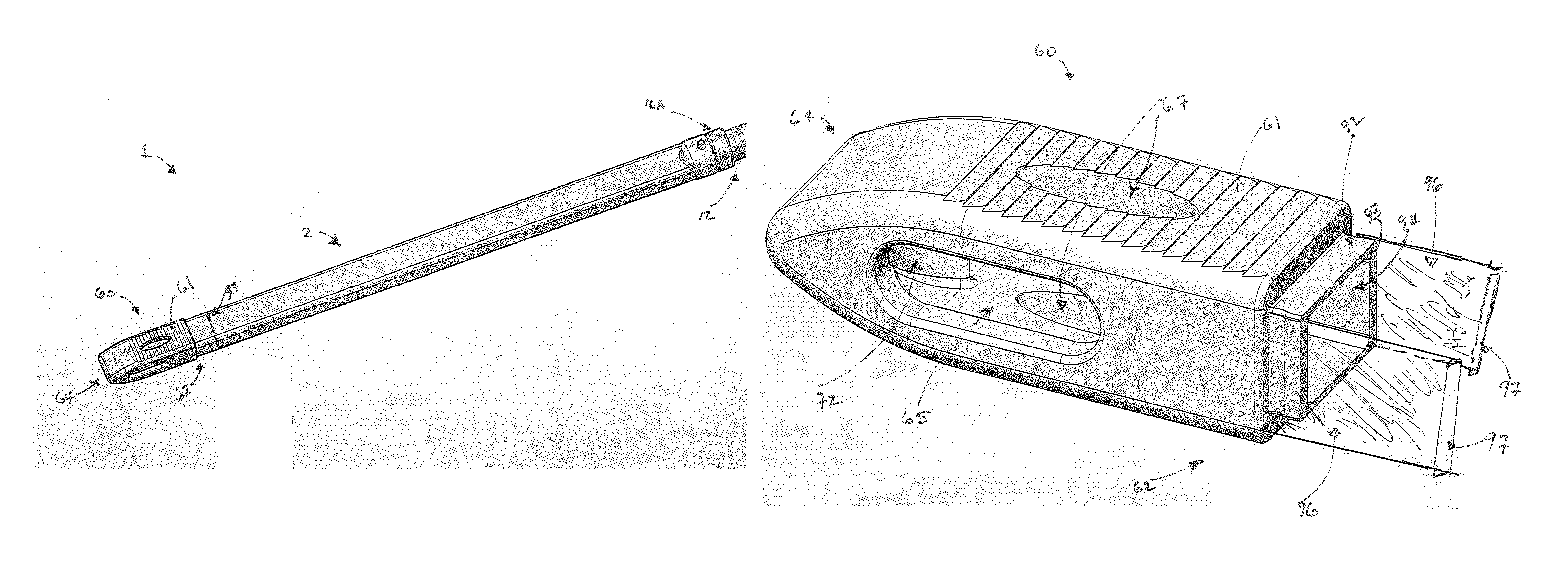
A minimally invasive interbody device assembly that includes an interbody device that restores the disc space height between two vertebrae and an instrument detachably coupled to the interbody device for positioning the device in the disc space and delivering bone material to the disc space that is distributed on both sides of the interbody device. The device is inserted into the disc space using the instrument in a direction so that the wide dimension of the device is substantially parallel to the body of the vertebrae. The device is then rotated by the instrument so that the wide dimension of the device becomes perpendicular to the vertebral body so as to cause the disc space height to be restored. Bone graft material is then forced down the instrument so that the bone graft material is distributed on both sides of the device. The instrument is then detached from the device.
Owner:THOMPSON MIS
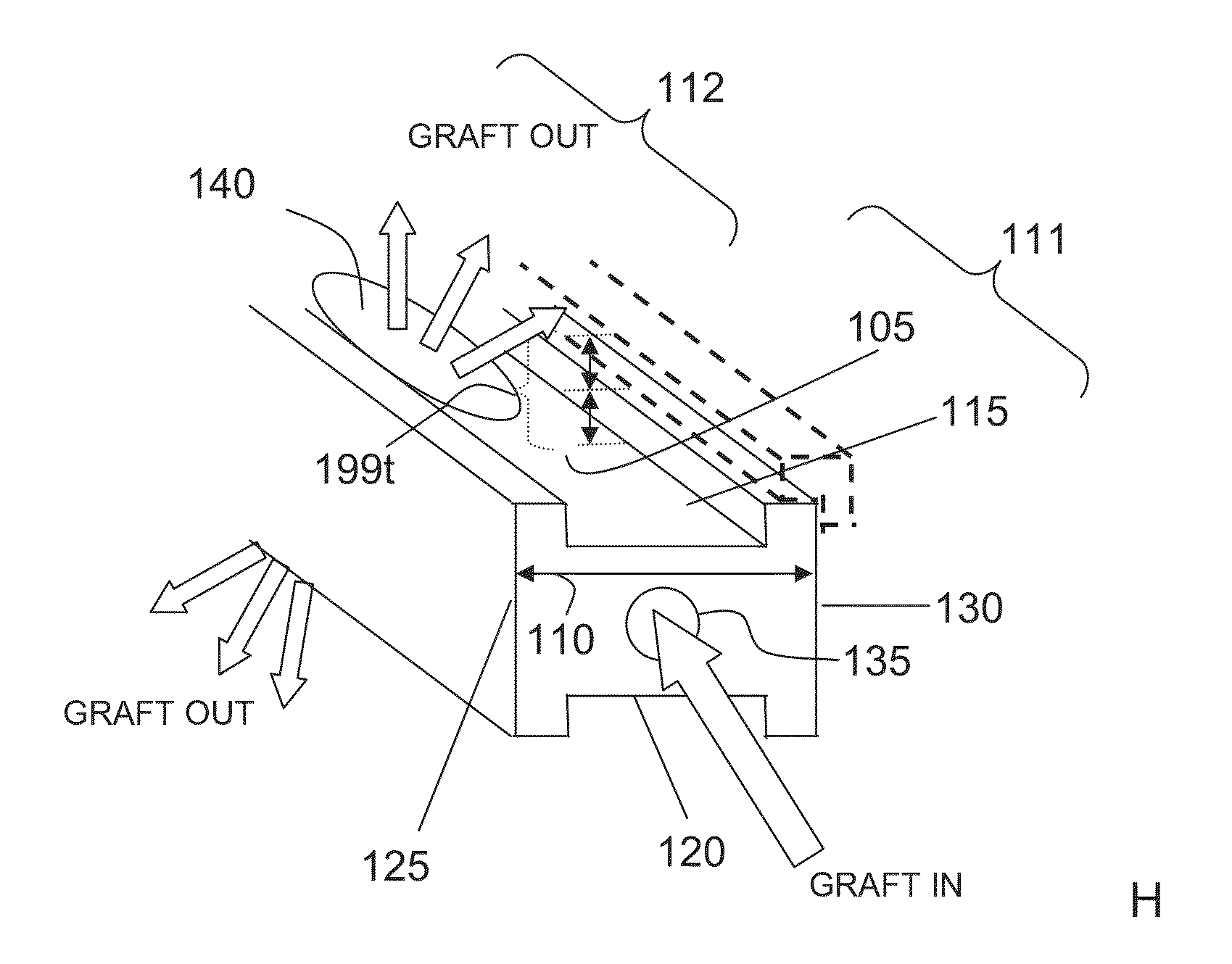
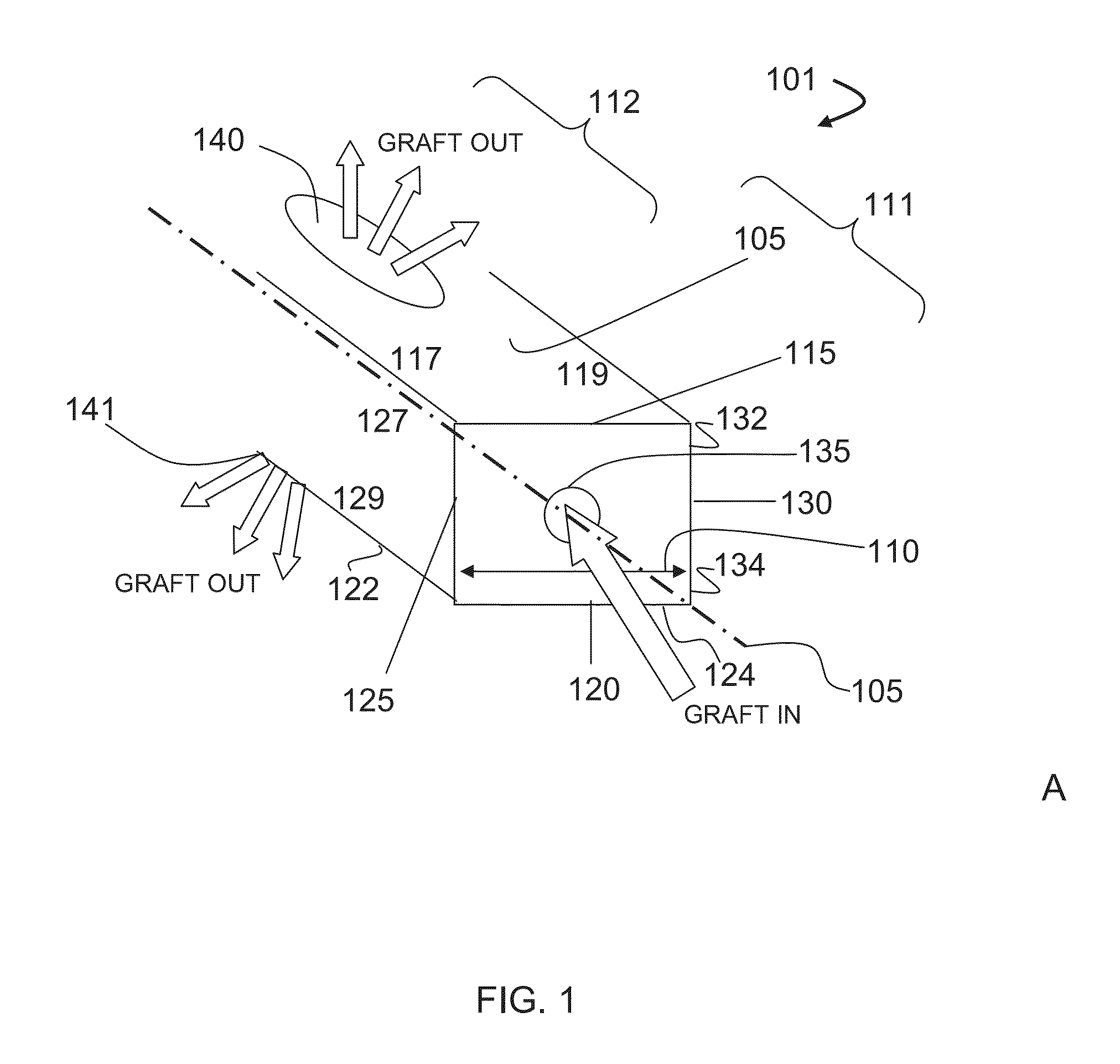
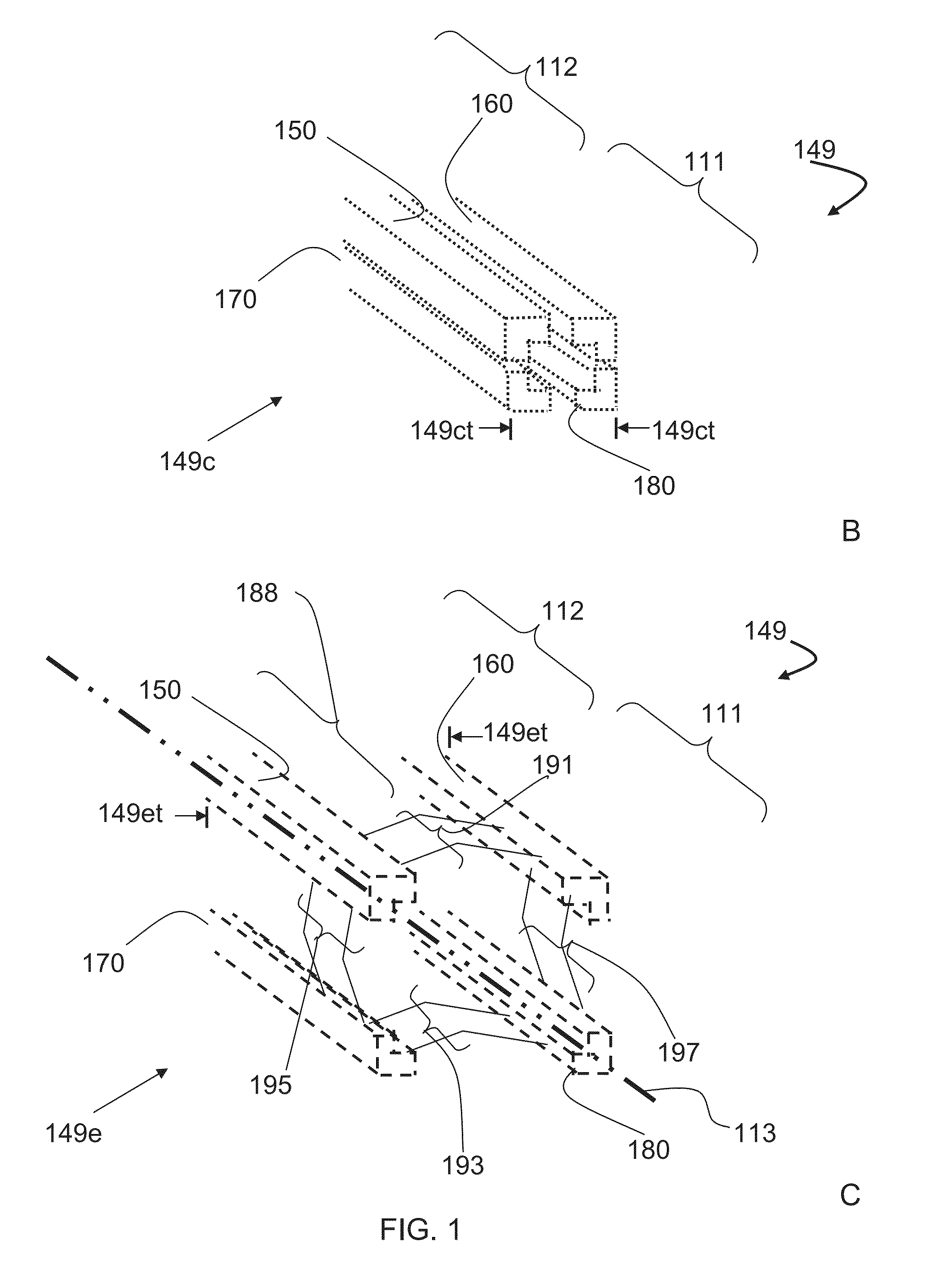
Bone graft distribution system
ActiveUS8663332B1Facilitate graft distributionBone implantSpinal implantsDistal portionDistribution system
A system for distributing bone graft material in an intervertebral disc space is provided having a central beam having a proximal portion having an end, a grafting portion having a top and a bottom, a distal portion having a end, a central beam axis, a graft distribution channel having an entry port at the end of the proximal portion, a top exit port at the top of the grafting portion, and a bottom exit port at the bottom of the grafting portion. These systems can also include a laterovertically-expanding frame operable for a reversible collapse from an expanded state into a collapsed state. The expanded state, for example, can be configured to have an open graft distribution window that at least substantially closes upon the reversible collapse.
Owner:INTEGRITY IMPLANTS INC
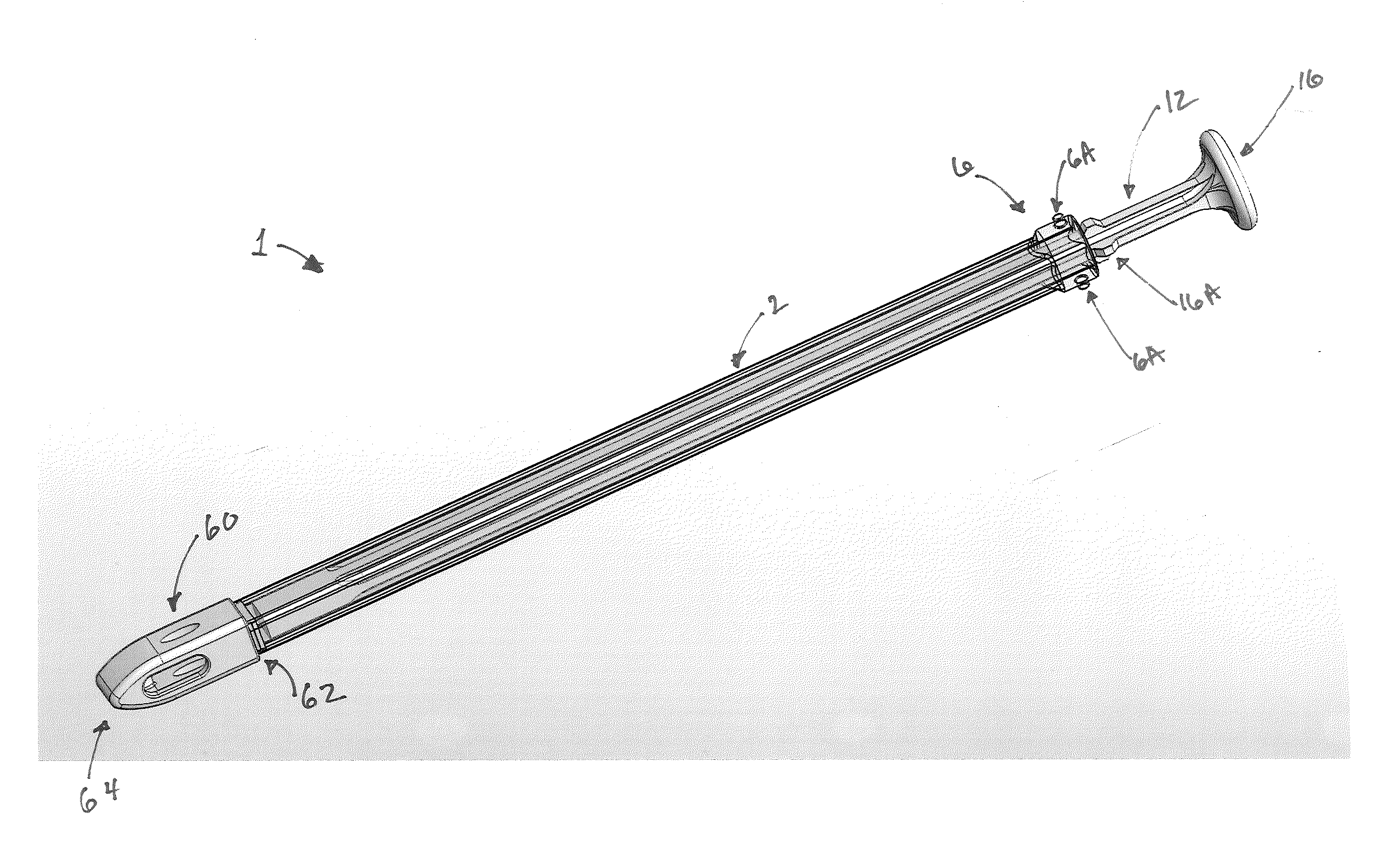
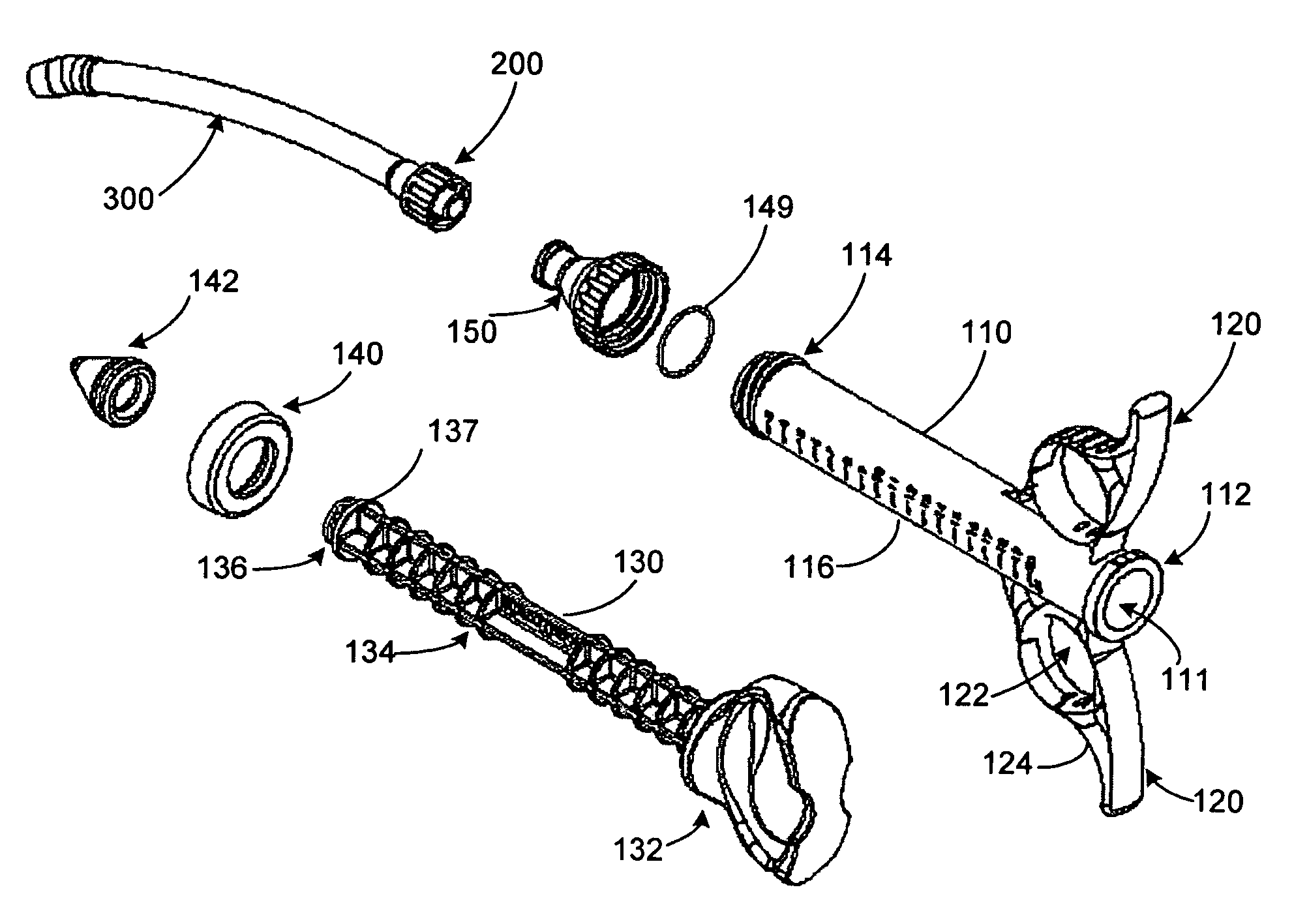
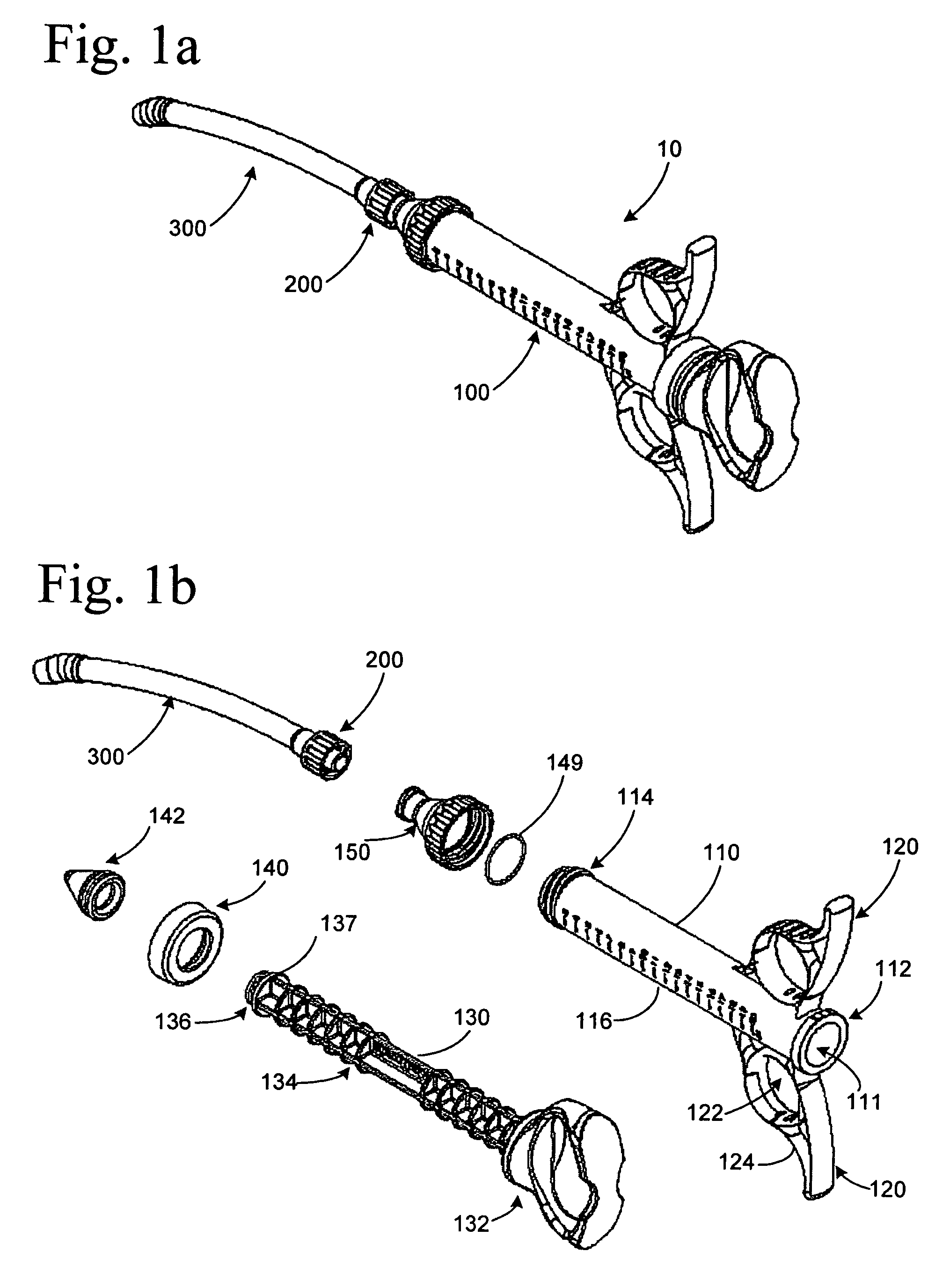
Graft syringe assembly
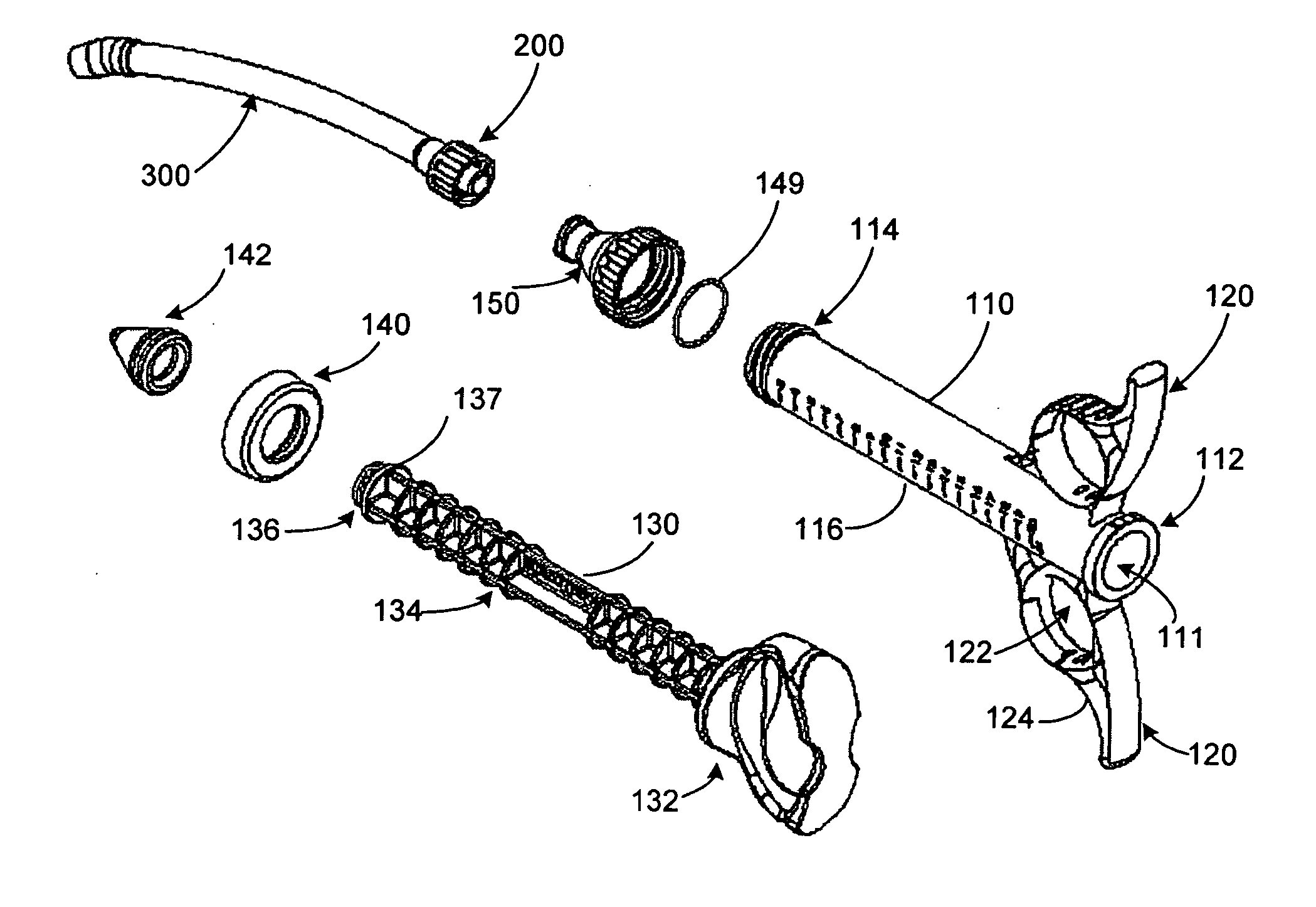
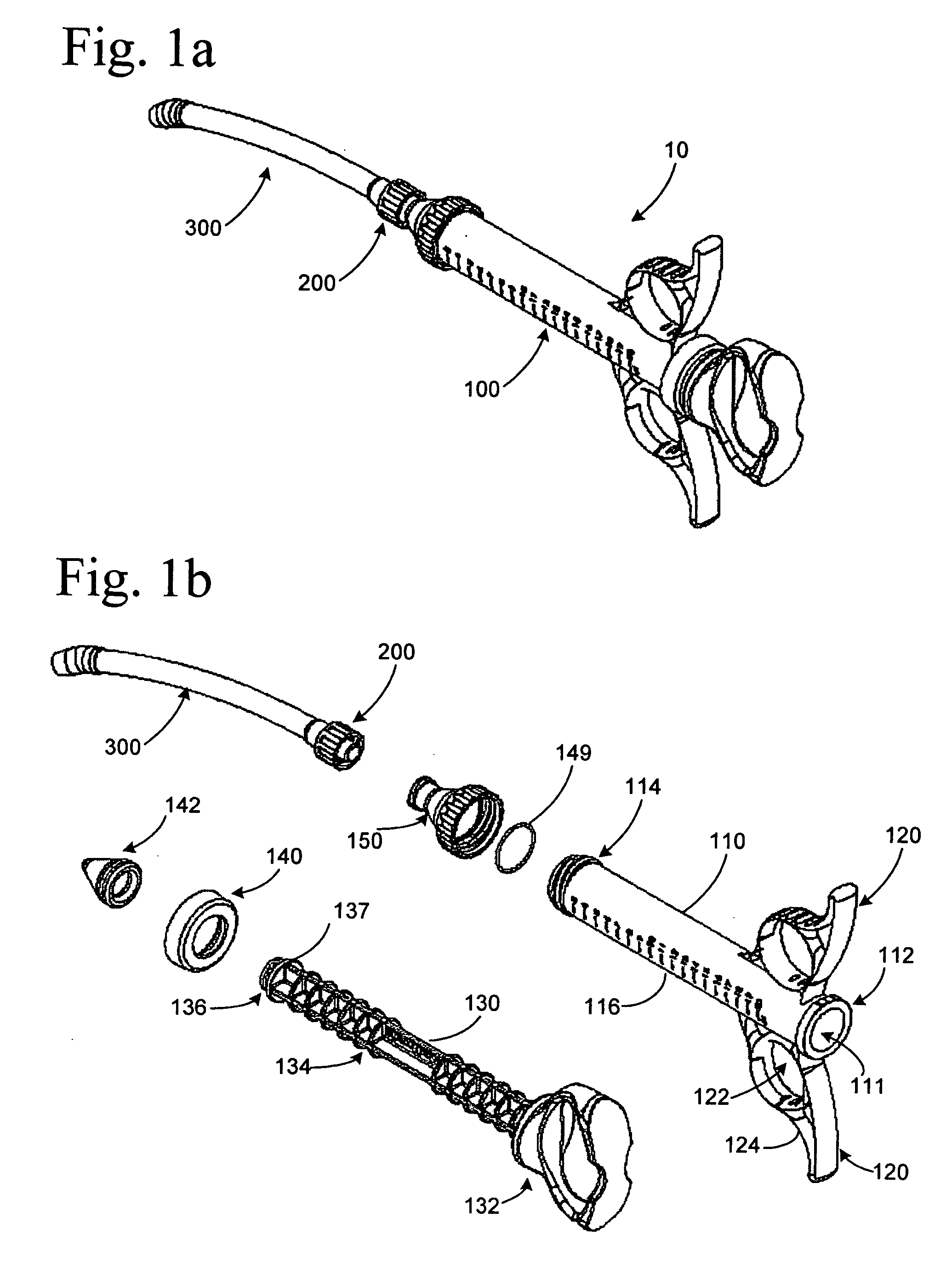
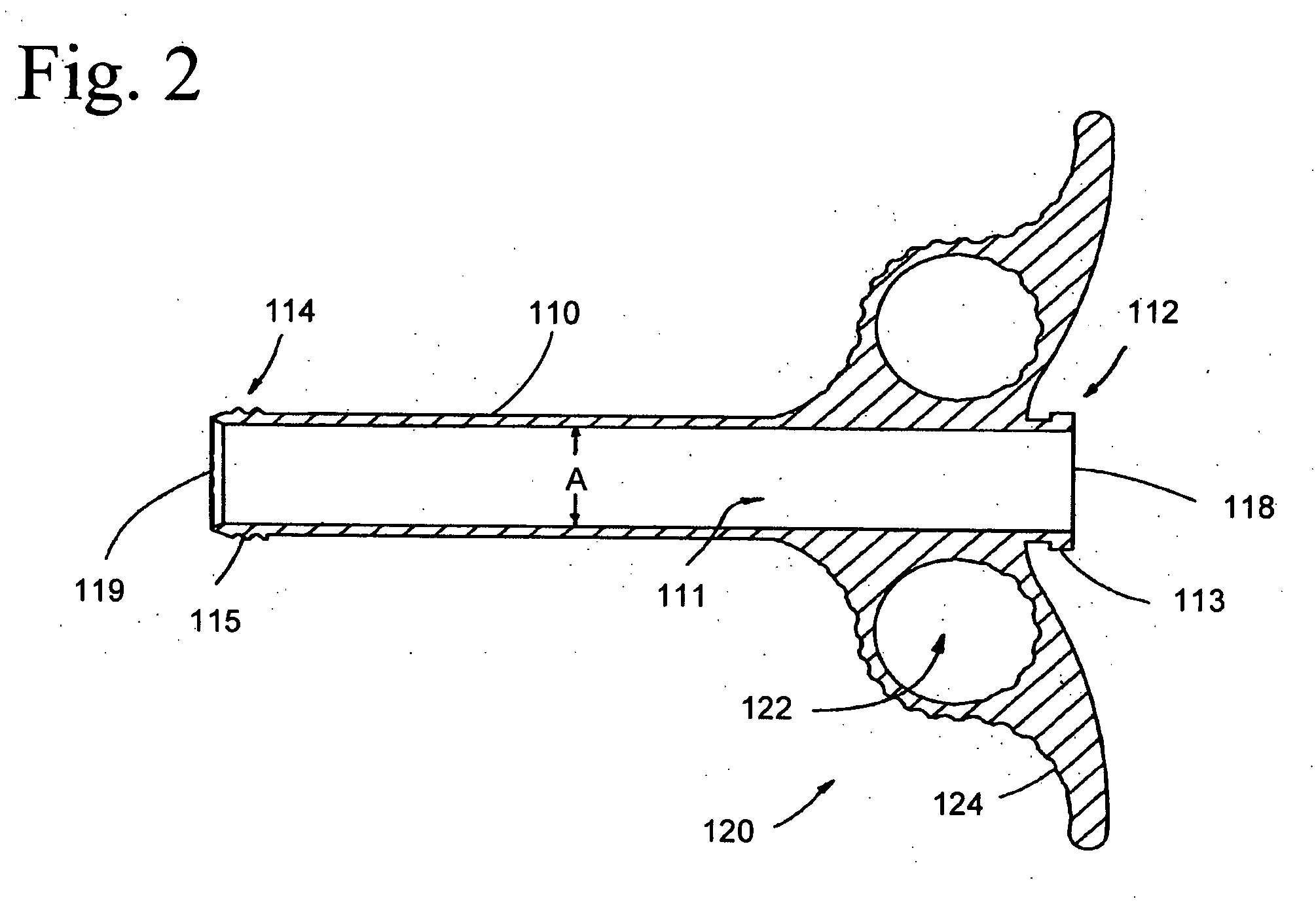
The invention relates, according to one embodiment, to a graft syringe assembly for delivering bone graft material is disclosed. The graft syringe assembly comprises a syringe subassembly including a syringe barrel having an inner chamber adapted for receiving bone graft material, a plunger adapted for expelling bone graft material from the inner chamber, the plunger slidably received within the inner chamber, and a syringe adapter coupled to the syringe barrel. The graft syringe assembly further comprises a connection subassembly coupled to the syringe adapter, and a delivery tube subassembly coupled to the connection subassembly, wherein the connection subassembly is configured to allow the delivery tube subassembly to rotate relative to the syringe subassembly.
Owner:WARSAW ORTHOPEDIC INC
Spinal fusion implants and devices and methods for deploying such implants
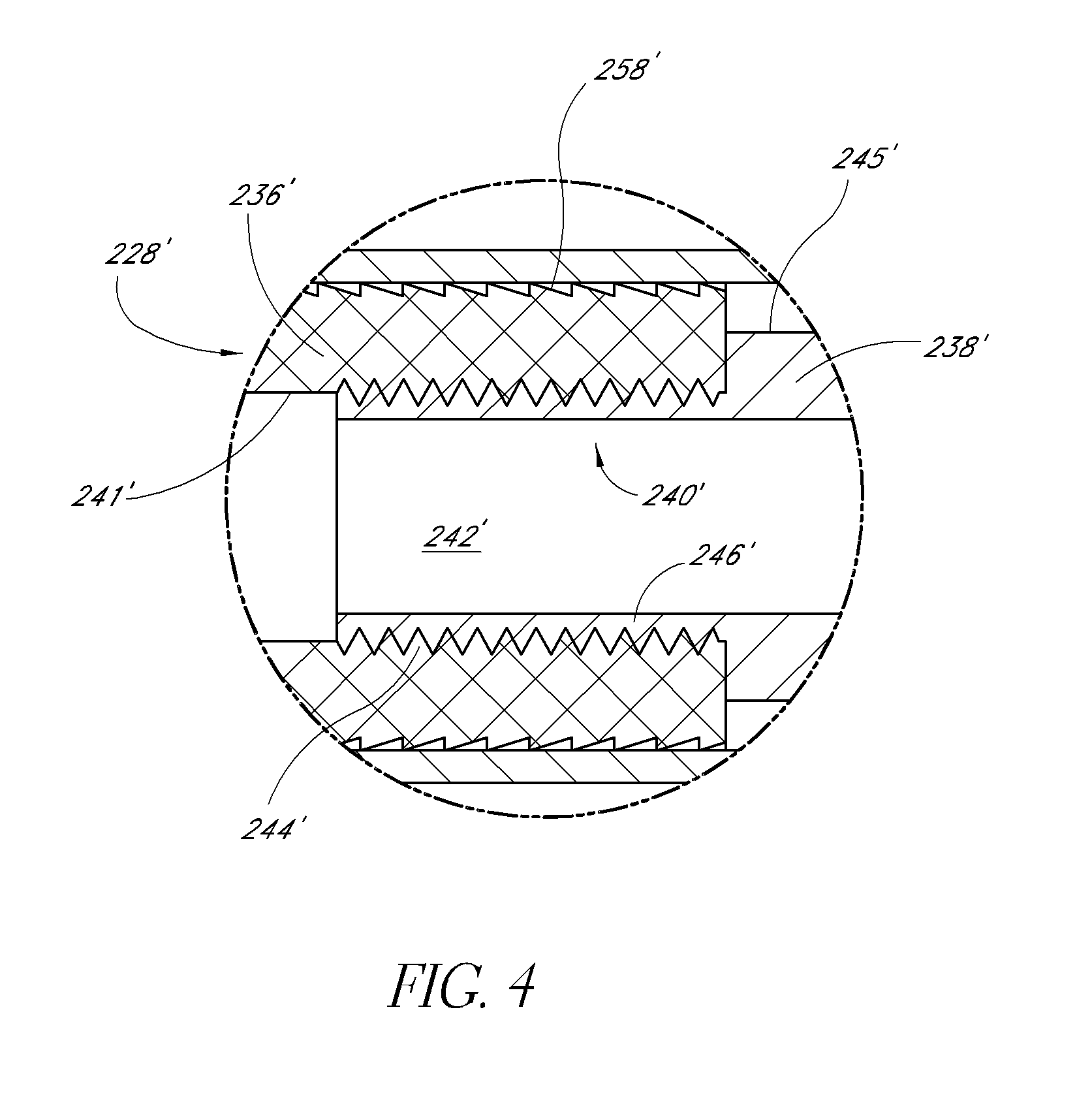
ActiveUS20140277481A1Add dimensionSpinal implantsOsteosynthesis devicesDistractionBone graft materials
Methods and apparatus are disclosed for distracting tissue. The devices and methods may include insertion of first and second elongated members into the space between two tissue layers, with an augmenting elongated member inserted therebetween to form a distraction device between the tissues to be distracted. The distraction device defines a generally annular configuration, with a locking member secured to one of the elongated members at a plurality of locations to maintain the distraction device in the generally annular configuration. The augmenting elongated member may be shorter than the first and second elongated members such that a window is defined between the proximal and distal ends of the augmenting elongated member when the distraction device and the first and second elongated members are in the generally annular configuration. Bone graft material or bone filler may be introduced into the interior of the distraction device through the window.
Owner:SPINAL ELEMENTS INC
Methods and Systems for Interbody Implant and Bone Graft Delivery
InactiveUS20120065613A1Reduces approach related morbidityEasy to controlMedical devicesIntravenous devicesBone graft materialsCatheter
A bone graft system for providing bone graft material to a site of interest includes a multiple unit bone graft material loading device and a conduit. The multiple unit bone graft material loading device is configured to accept a plurality of pre-formed bone graft material units and includes a plurality chambers configured to accept a pre-formed bone graft unit. The conduit includes a first opening and second opening. The first opening is operably connected with the multiple unit bone graft material loading device to accept bone graft material from the multiple unit bone graft material loading device. The second opening is configured to deliver bone graft material to a site of interest.
Owner:THOMPSON MIS
Minimally Invasive Interbody Device
ActiveUS20080172127A1Restore heightDisc space heightInternal osteosythesisEar treatmentBone graft materialsVertebra
An interbody device that restores the disc space height between two vertebrae during spinal fusion surgery. The device includes a center plate surrounded by a perimeter portion that combine to have a relatively flat configuration in one dimension and relatively wide configuration in a perpendicular dimension. After the disc space has been cleared, the device is inserted into the disc space in a direction so that the wide dimension of the device is substantially parallel to the body of the vertebrae. The device is then rotated so that the wide dimension of the device becomes perpendicular to the vertebral body so as to cause the disc space height to be restored. Bone graft material is then introduced through a fill tube coupled to the device so that the bone graft material is distributed on both sides of the center plate and into the disc space.
Owner:THOMPSON MIS
Fusion cage with combined biological delivery system
ActiveUS20120136442A1Accurate maneuveringConvenient to accommodateBone implantSpinal implantsSurgical siteBiological materials
The present invention relates to an apparatus and method for near-simultaneous and integrated delivery of bone graft material during the placement of surgical cages or other medical implants in a patient's spine. The integrated fusion cage and graft delivery device according to various embodiments delivers and disperses biologic material through a fusion cage to a disc space and, without withdrawal from the surgical site, may selectably detach the fusion cage for deposit to the same disc space. The integrated fusion cage and graft delivery device is formed such that a hollow tube and plunger selectively and controllably place bone graft material and a fusion cage in or adjacent to the bone graft receiving area.
Owner:SPINAL SURGICAL STRATEGIES INC
Resorbable bone graft materials
Ceramic materials operable to repair a defect in bone of a human or animal subject comprising a porous ceramic scaffold having a bioresorbable coating, and a carrier comprising denatured demineralized bone. The ceramic may contain a material selected from the group consisting of hydroxyapatite, tricalcium phosphate, calcium phosphates, calcium carbonates, calcium sulfates, and combinations thereof. The compositions may also contain a bone material selected from the group consisting of: bone powder, bone chips, bone shavings, and combinations thereof. The bioresorbable coating may be, for example, demineralized bone matrix, gelatin, collagen, hyaluronic acid, chitosan, polyglycolic acid, polylactic acid, polypropylenefumarate, polyethylene glycol, or mixtures thereof.
Owner:BIOMET MFG CORP
Graft syringe assembly
The invention relates, according to one embodiment, to a graft syringe assembly for delivering bone graft material is disclosed. The graft syringe assembly comprises a syringe subassembly including a syringe barrel having an inner chamber adapted for receiving bone graft material, a plunger adapted for expelling bone graft material from the inner chamber, the plunger slidably received within the inner chamber, and a syringe adapter coupled to the syringe barrel. The graft syringe assembly further comprises a connection subassembly coupled to the syringe adapter, and a delivery tube subassembly coupled to the connection subassembly, wherein the connection subassembly is configured to allow the delivery tube subassembly to rotate relative to the syringe subassembly.
Owner:WARSAW ORTHOPEDIC INC
Fusion cage with combined biological delivery system
ActiveUS9186193B2Reduce the risk of infectionReduce the possibilityBone implantJoint implantsSurgical siteBiological materials
The present invention relates to an apparatus and method for near-simultaneous and integrated delivery of bone graft material during the placement of surgical cages or other medical implants in a patient's spine. The integrated fusion cage and graft delivery device according to various embodiments delivers and disperses biologic material through a fusion cage to a disc space and, without withdrawal from the surgical site, may selectably detach the fusion cage for deposit to the same disc space. The integrated fusion cage and graft delivery device is formed such that a hollow tube and plunger selectively and controllably place bone graft material and a fusion cage in or adjacent to the bone graft receiving area.
Owner:SPINAL SURGICAL STRATEGIES INC
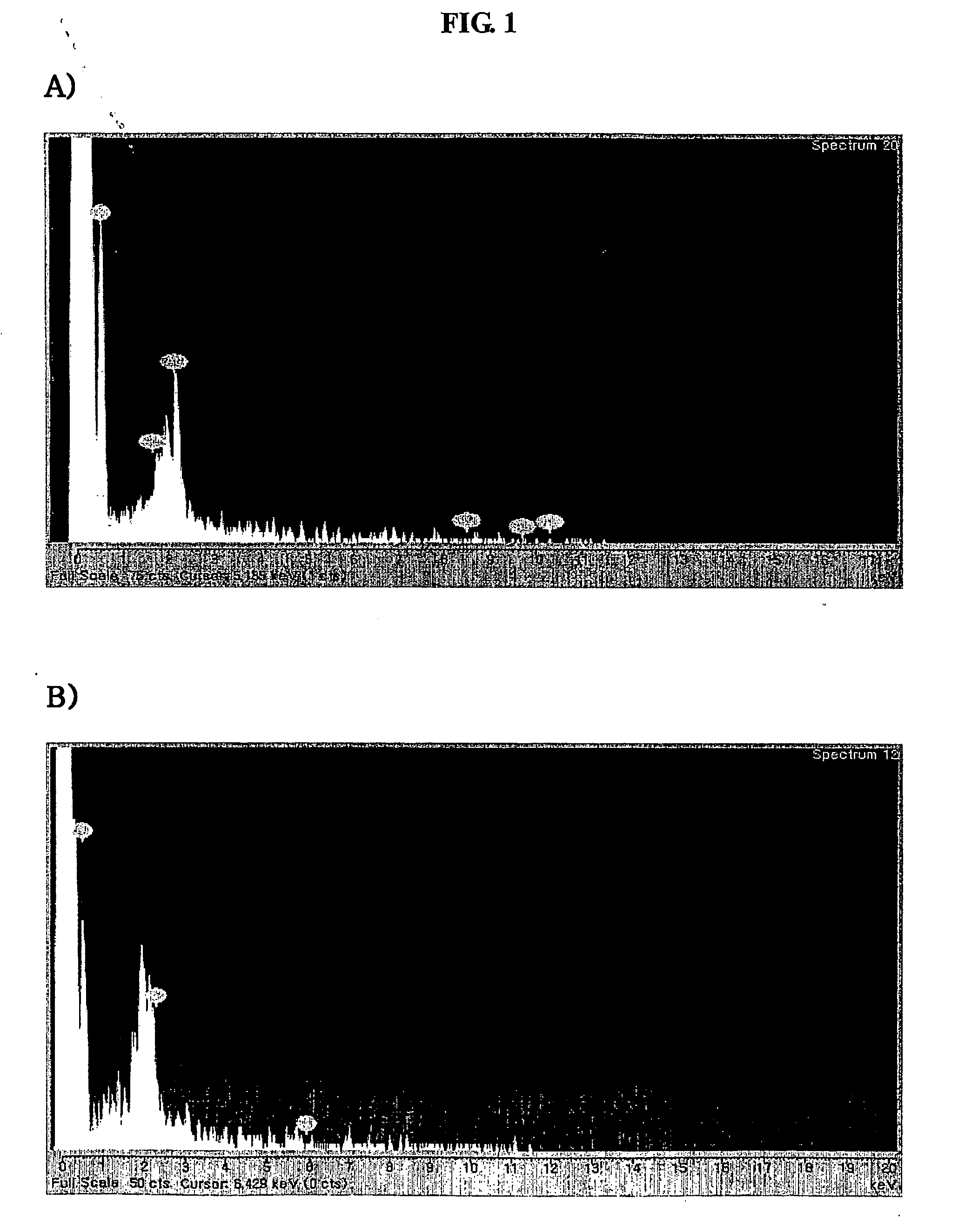
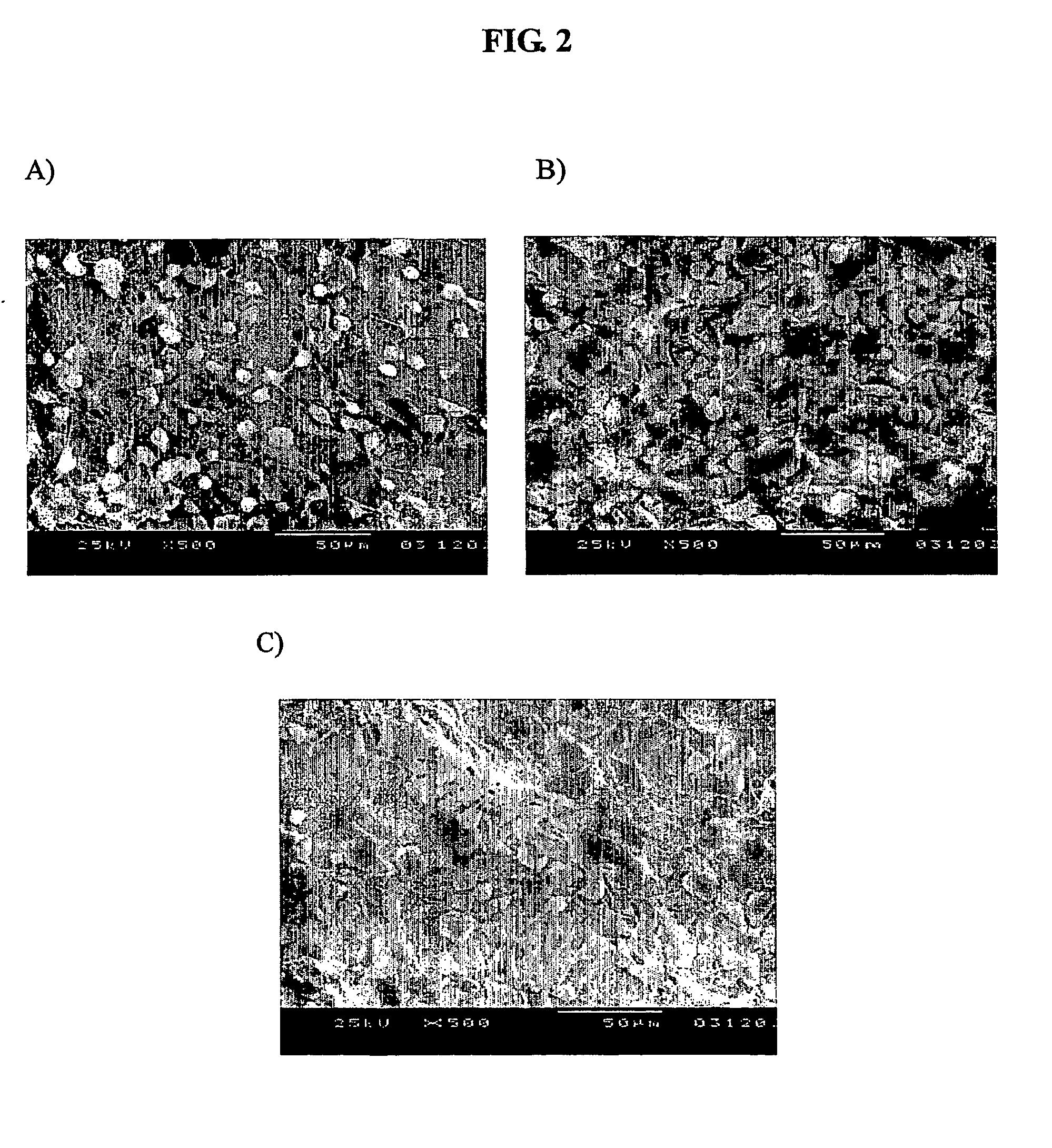
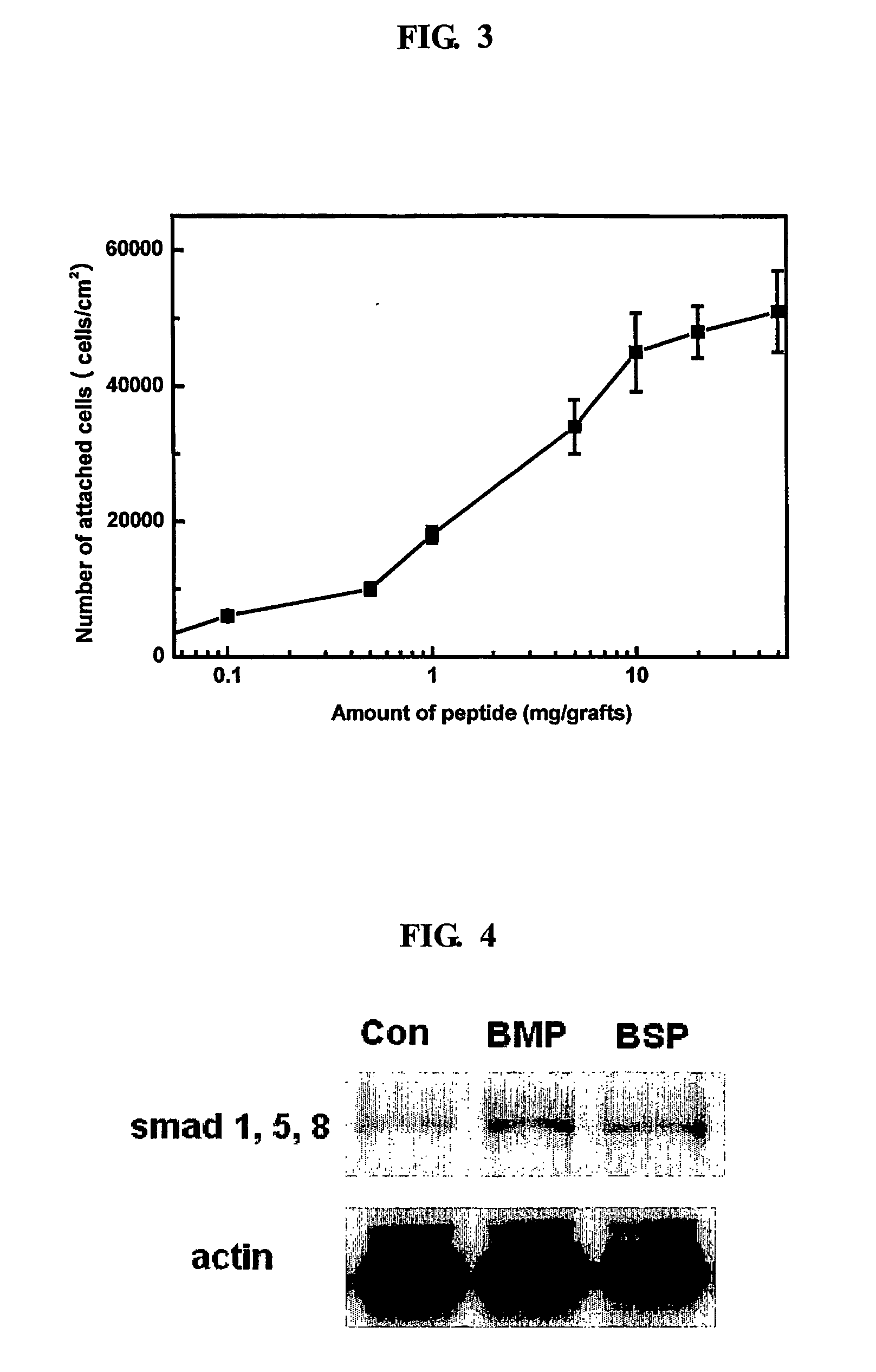
Bone graft and scaffolding materials immobilized with osteogenesis enhancing peptides on the surface
ActiveUS20070160681A1Improve efficiencyEasy to fixCoffee millsAnimal cellsSurgical operationCell adhesion
The present invention relates to a bone graft material and a scaffold for tissue engineering applications, which have an osteogenesis-promoting peptide immobilized on the surface. More particularly, the invention relates to a bone graft material and a scaffold for tissue engineering applications, which have a cell adhesion-inducing peptide and / or tissue growth factor-derived peptide immobilized on the surface. By the osteogenesis-promoting peptide immobilized on the surface, the inventive bone graft material and scaffold for tissue engineering applications can promote the transition, proliferation and differentiation of cells associated with regeneration, and eventually maximize the regeneration of tissue. Moreover, the peptide immobilized on the surface has low molecular weight, indicating a reduced risk of immune responses upon its application in the body, and can be present in a stable form within the body, thus showing lasting effects. Accordingly, the peptide makes it expedient to perform surgical operations for the regeneration of periodontal tissue, alveolar bone and other bone tissues, and will show high therapeutic effect.
Owner:SEOUL NAT UNIV R&DB FOUND
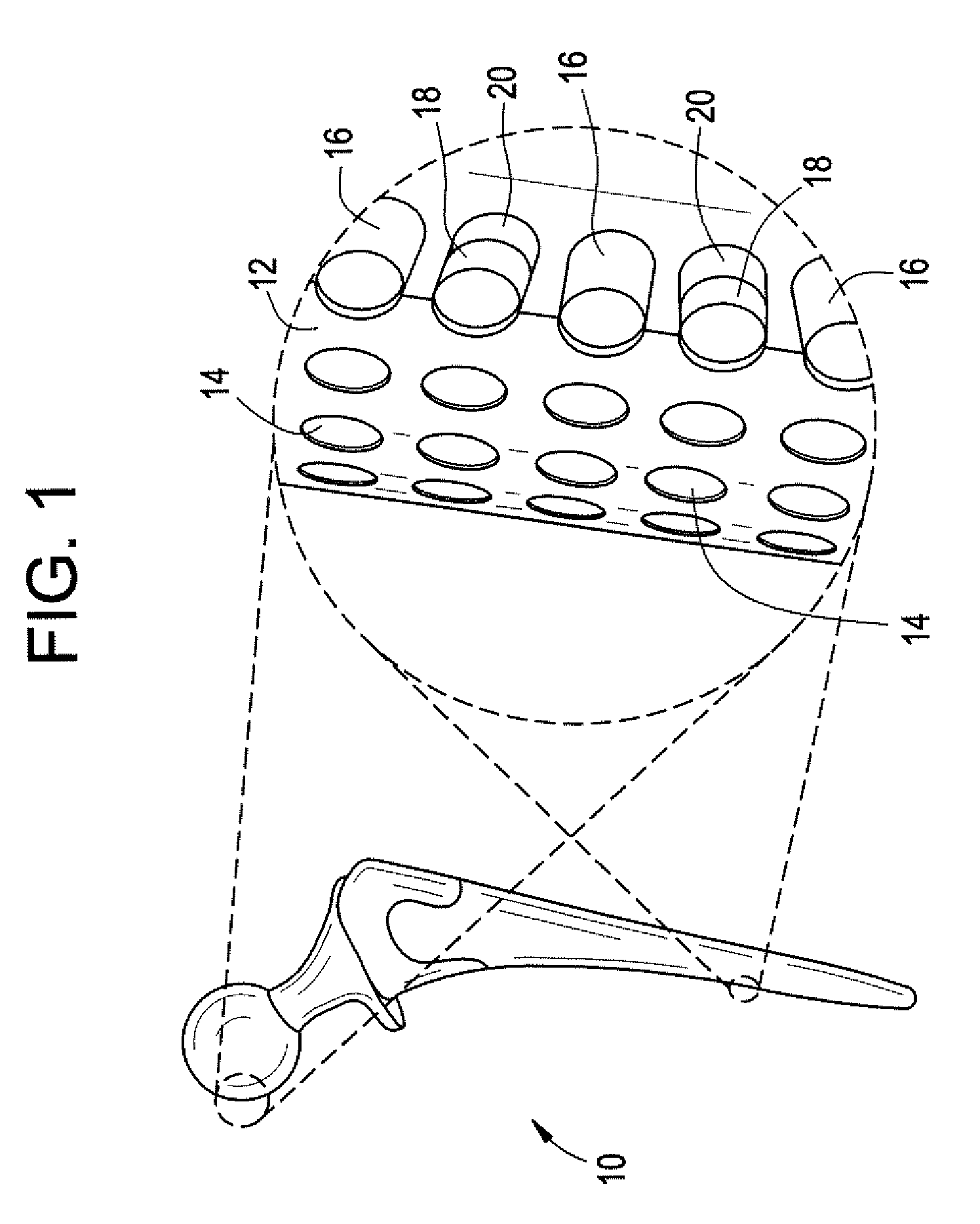
Spinal stabilization device and methods
Implant devices in the form of a vertebral body replacement for implantation within an intervertebral space between adjacent vertebrae is disclosed for immobilization and support of the adjacent vertebrae in a desired spatial relationship and for promotion of fusion by the adjacent vertebrae. Instruments for insertion and implantation of the implant devices and of fusion material such as bone graft material are disclosed. Methods are disclosed for implantation of the implant devices.
Owner:XTANT MEDICAL HLDG INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com