Demineralized bone-derived implants
a bone-derived implant and demineralization technology, applied in the field of bone-derived implants, can solve the problems of limiting the efficacy of these implants, not always possible or even desirable to use an autograft, and additional patient discomfort during rehabilitation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
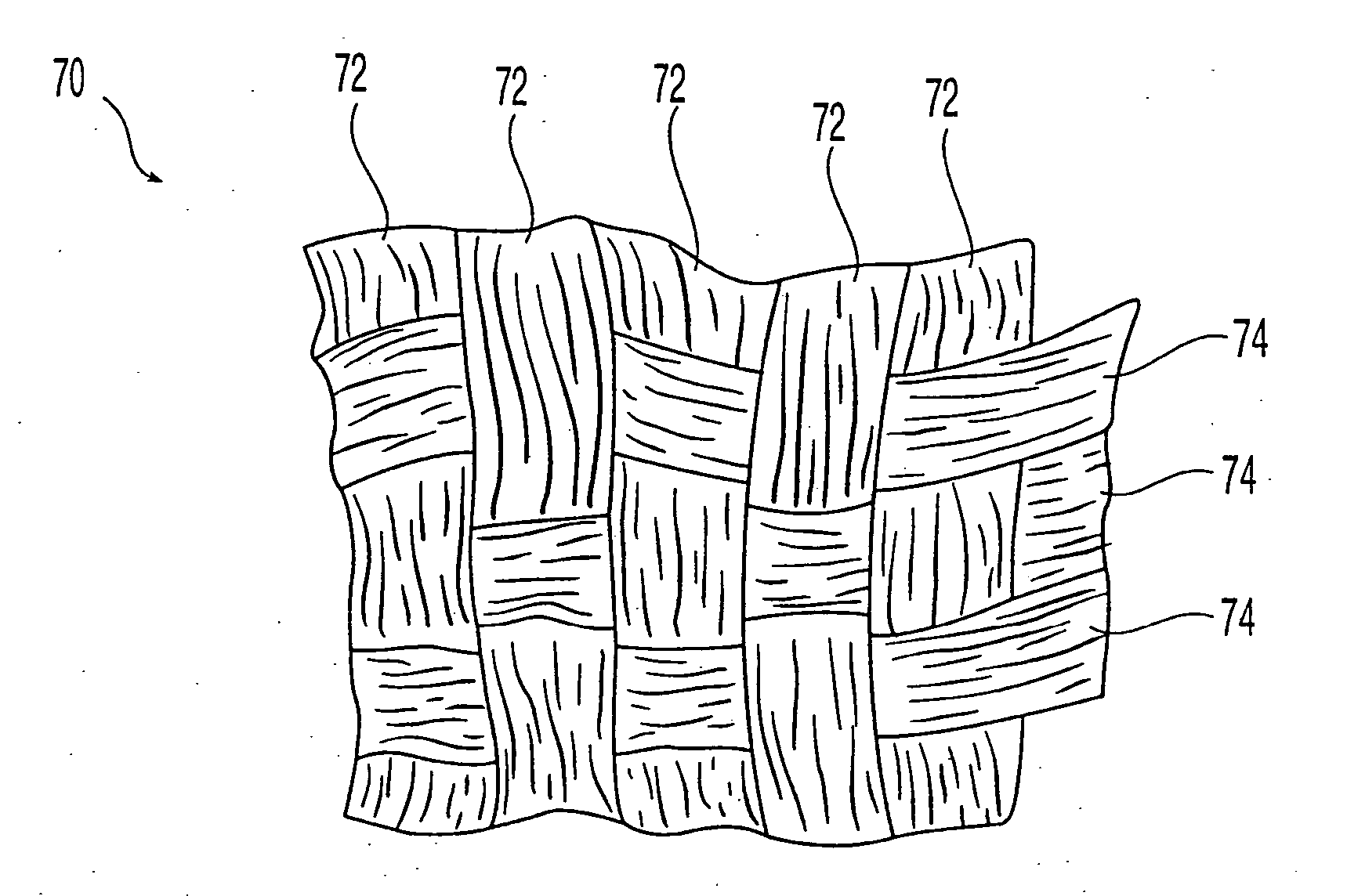
[0047] The present invention in one embodiment is directed to an implantable bone sheet that exhibits semi-pliable properties over portions of the sheet, while exhibiting semi-rigid properties over other portions. The variation in properties is achieved by the selective demineralization of bone preferably selected from a femur, tibia, humerus, fibula, ulna, and radius. The terms “demineralization,”“demineralized” and “at least partially demineralized” as used herein are intended to refer to fully demineralized bone or partially demineralized bone. The term “fully demineralized” refers to bone where the minerals have been substantially completely removed from the bone whereas the term “partially demineralized” refers to bone where at least some portion of the minerals have been removed. As will become apparent, the degree of demineralization will depend upon the characteristics sought to be achieved in the implant.
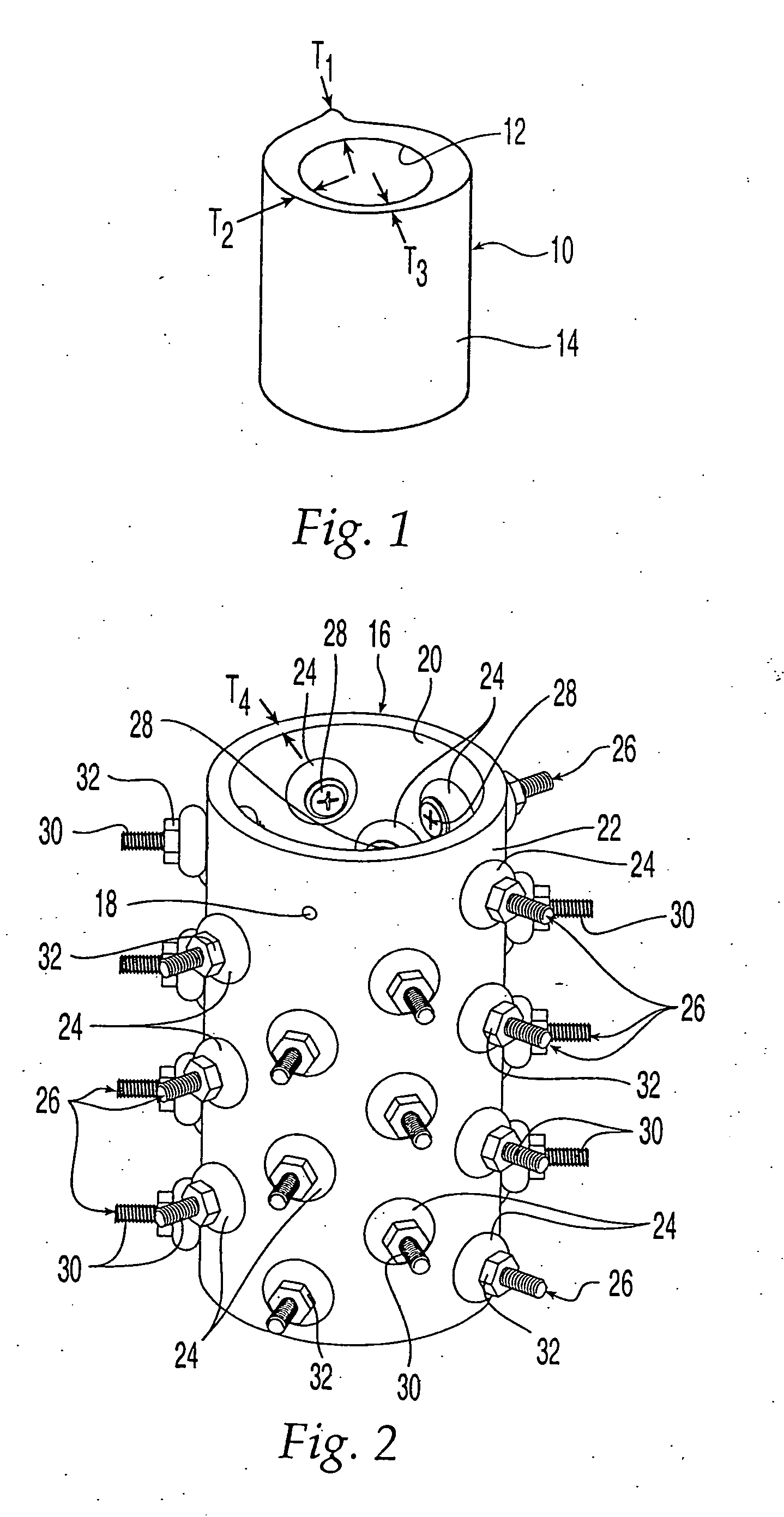
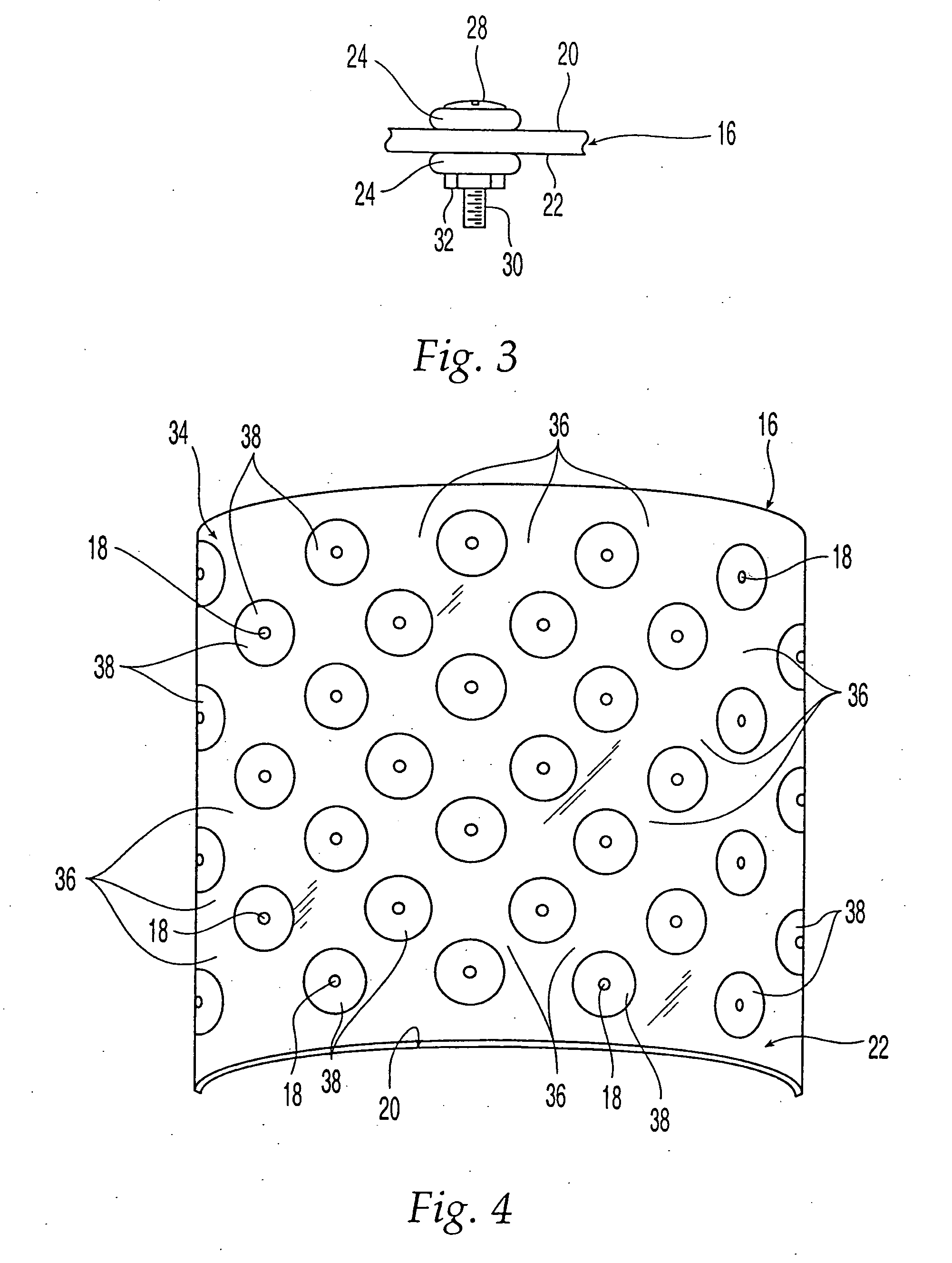
[0048] Turning to FIG. 1, a bone section 10 of a femur has an inner s...
PUM
| Property | Measurement | Unit |
|---|---|---|
| width | aaaaa | aaaaa |
| width | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com