STEM CELL MICROPARTICLES AND miRNA
a technology of stem cells and microparticles, which is applied in the field of stem cell microparticles and mirna, can solve the problems of low regenerative capacity of damaged central nervous system (cns) tissue, no convincing evidence for direct long-term effect of transplanted stem cells, and potential safety risks of tumour or ectopic tissue formation, so as to reduce migration, promote differentiation of tumours, and reduce the effect of stem cell marker nestin
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
mes that Inhibit Cell Migration
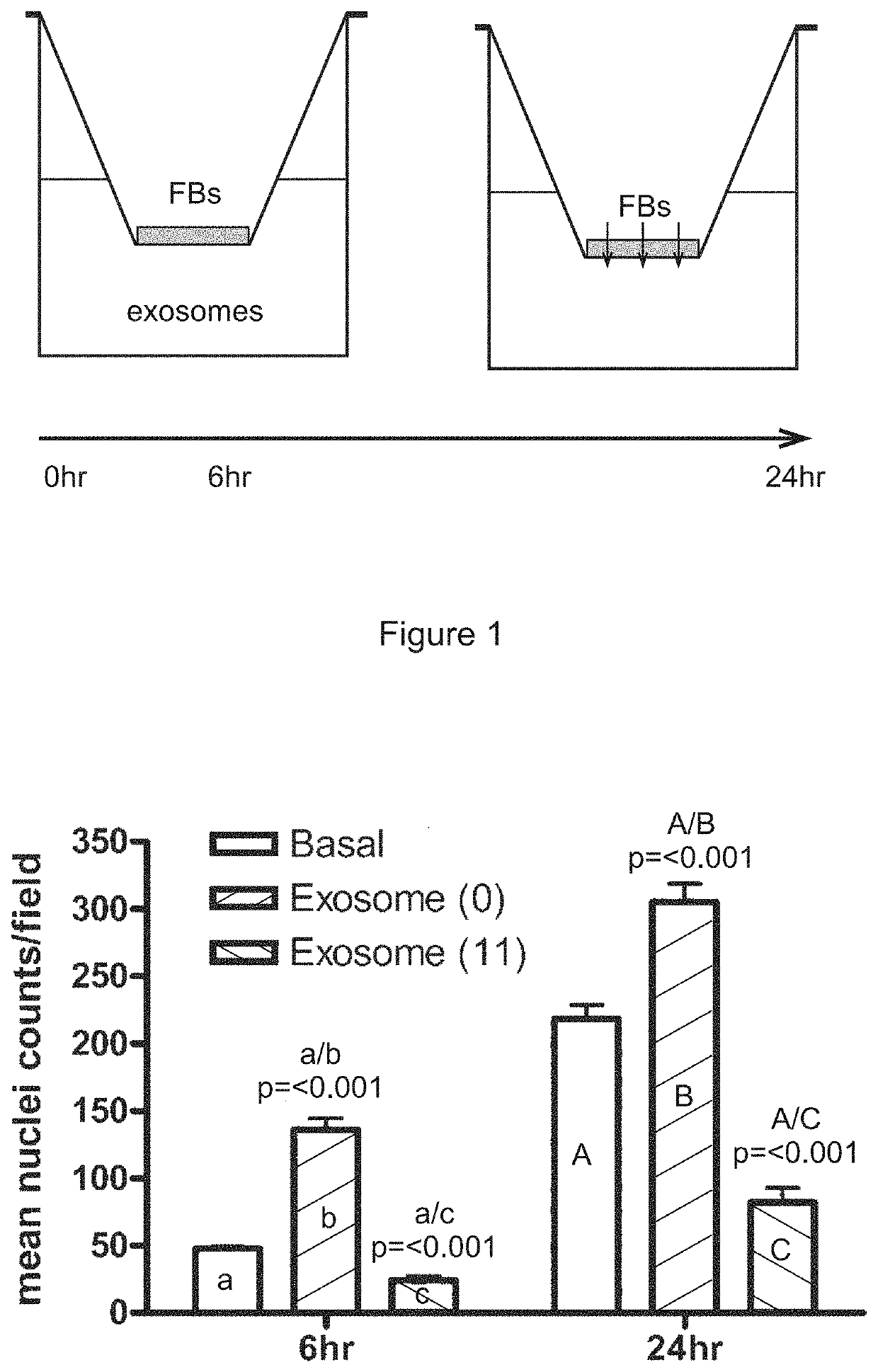
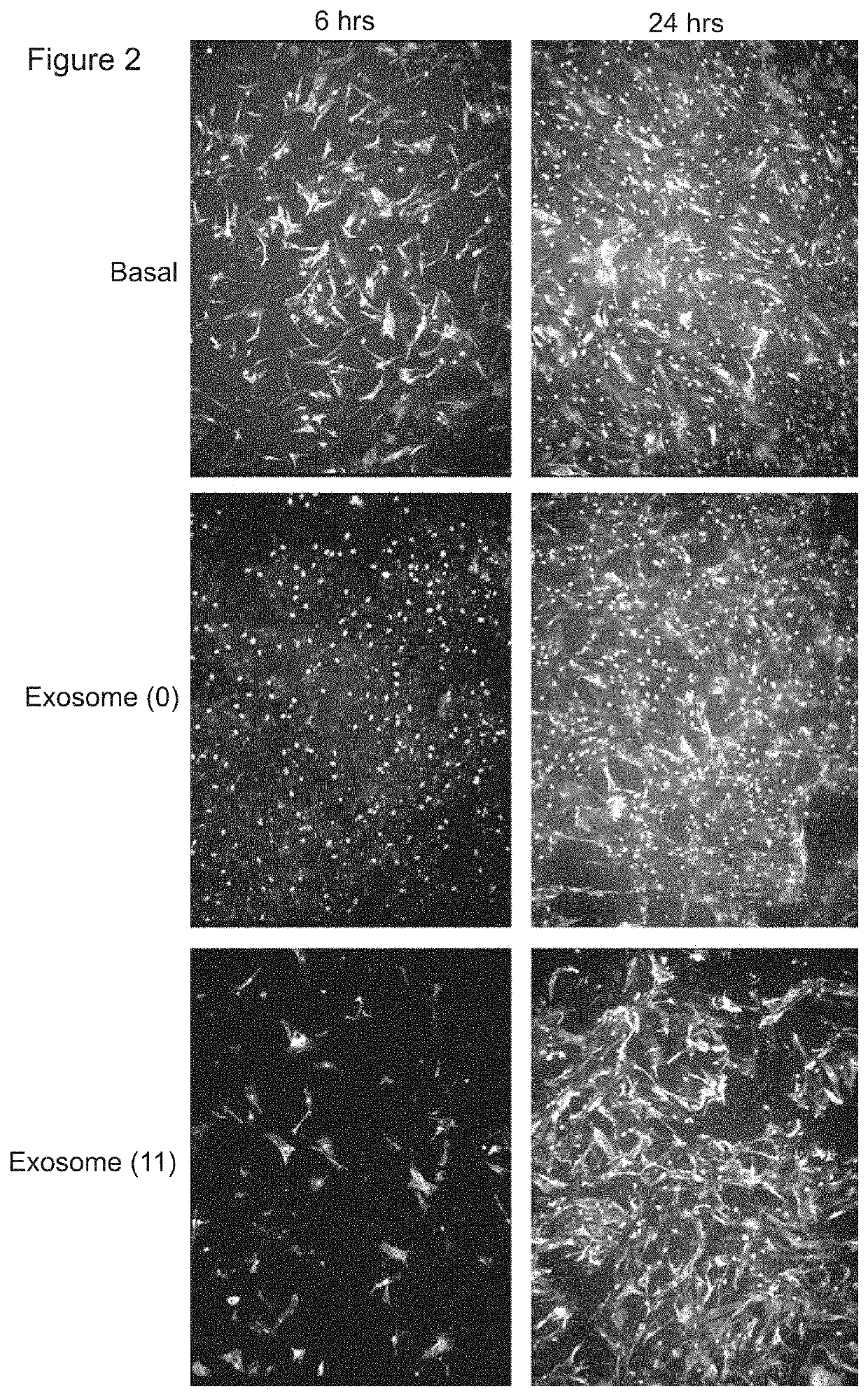
[0366]A transwell assay was used to study the migratory response of human dermal fibroblasts to different populations of exosomes. Experiments were performed in triplicate. 200,000 human dermal fibroblast cells (“FBs”) were placed on the upper layer of a cell permeable membrane (8 μm pore size; 24-well plate) and a solution (basal medium) containing or lacking 20 μg / ml exosomes was placed in contact with the underside of the cell permeable membrane (FIG. 1, top panel). The exosomes were collected from CTX0E03 cells cultured for 0 weeks (“0”) or 11 weeks (“11”) in an Integra CeLLine AD1000 multi-chamber bioreactor. Following an incubation period (6 or 24 hours; control: 0 hours), the human dermal fibroblast cells that migrated through the membrane were stained (using a fluorescent-dye conjugated anti-actin antibody and Hoechst Fluorescent Stain for nuclei) and counted (six random microscope fields per sample) as an indicator of the cells' migratory resp...
example 2
Isolated from the Medium of NSCs Cultured for 2 or 6 Weeks Promote Fibroblast Migration
Method—
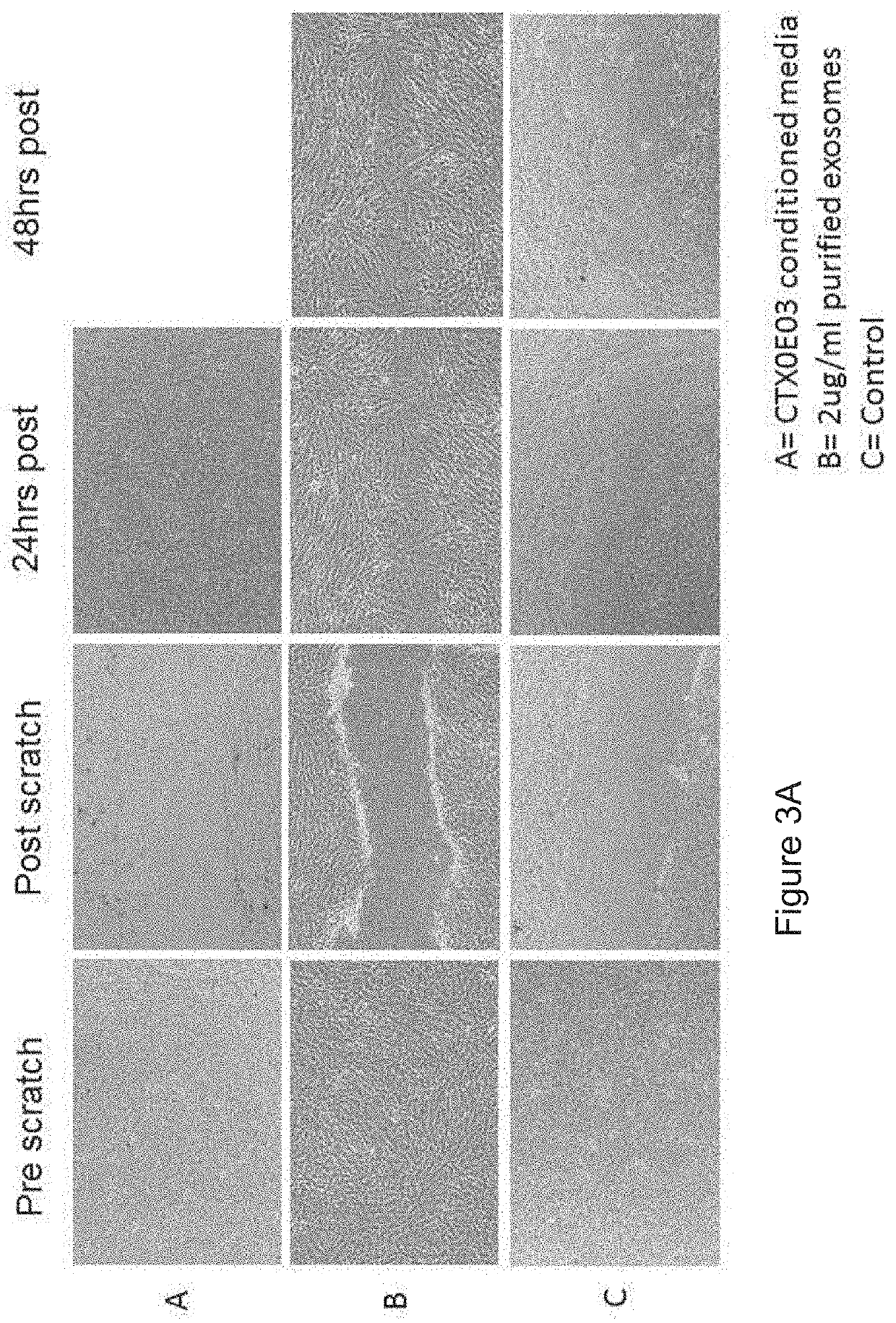
[0371]Wound closure / scratch assay[0372]Seed 0.25×106 NHDF (normal human dermal fibroblasts) per well of a 12 well plate and allow to become confluent (24 hours)[0373]Remove growth factors for 24 hrs[0374]Remove cells (scratch) and incubate with exosomes / conditioned media[0375]Image effected area over 48 hrs[0376]Estimate area using Image J
Results
[0377]
TABLE 2Wound closure / scratch assay representing the migration activity of normal human dermal fibroblasts (NHDF) cultured in CTX0E03 conditioned media or upon the addition of purified exosomes.Wound closure (%)0 h24 h48 hCTX0E03 conditioned media 0% 100%2 ug / ml exosomes0%95.4% 100%Control0%48.1%49.7%
[0378]Wound closure was calculated as the area covered by cells in relation to the initial wound area, as determined at 0 h. Wound closure is expressed as the percentage of the initial wound area at time 0 h. These data are also shown, photographic...
example 3
oma Engraftment Assay—Destruction of Tumour Cells
[0384]U373 glioblastoma cells were pre-treated in vitro for 24 hours with exosomes isolated from CTX0E03 cells cultured for 11 weeks in an Integra CeLLine bioreactor before implantation into the striatum of Balb-C mice brains.
[0385]As shown in FIG. 19, the exosome-treated glioblastoma cells did not engraft into the striatum. Histopathology demonstrated the presence of necrotic U373 cell bodies at the site of implantation and evidence of gliosis—a host cellular immune response.
[0386]These data suggest utility of these exosomes in the treatment of cancer, by promoting the destruction of a tumour by the immune system, particularly a tumour of the CNS such as a glioblastoma.
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com