Flexible elongated chain implant and method of supporting body tissue with same
a flexible chain and chain technology, applied in the field of implants, can solve the problems of prolonged disability, limited treatment options of compression fractures and related deformities, and repositioning fractured bones, and achieve the effect of sufficient flexibility and sufficient strength
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
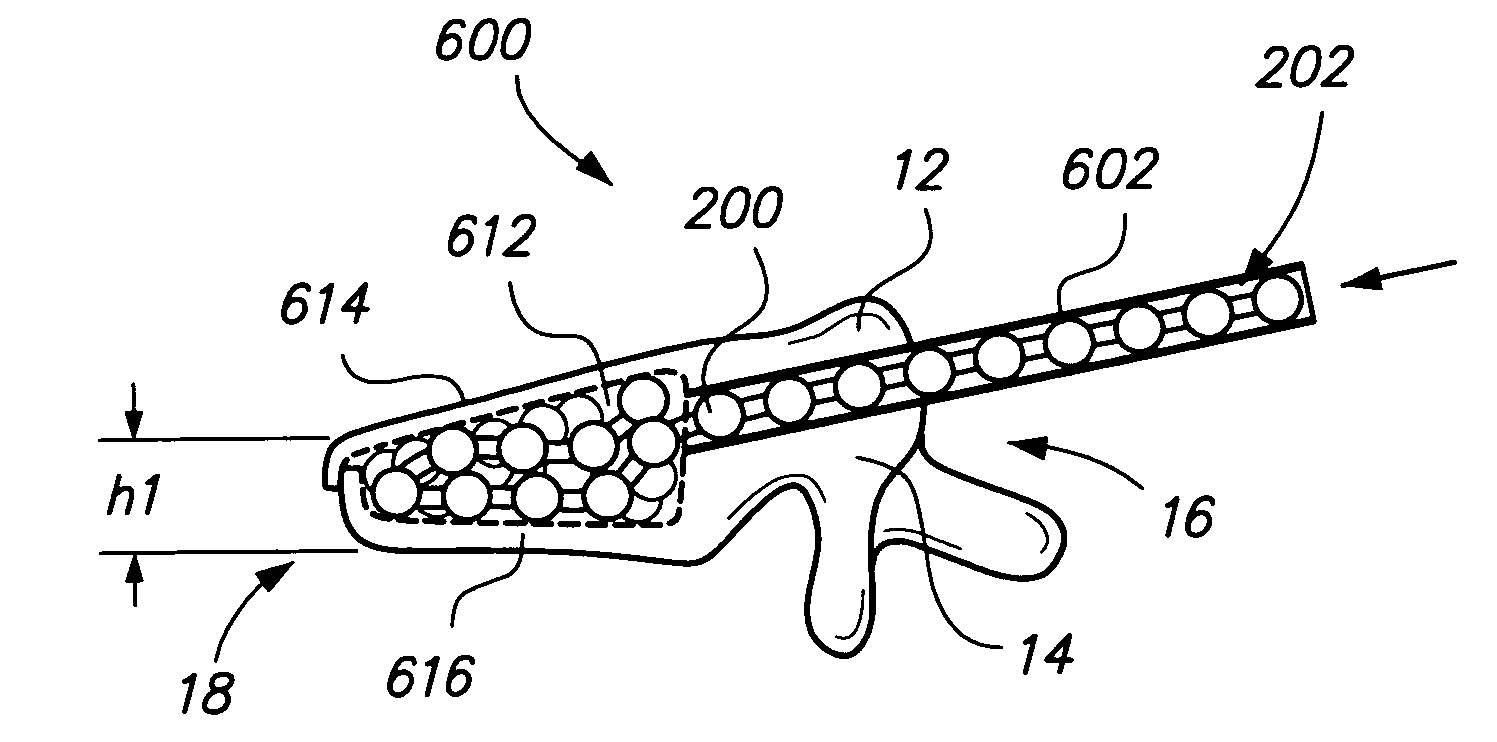
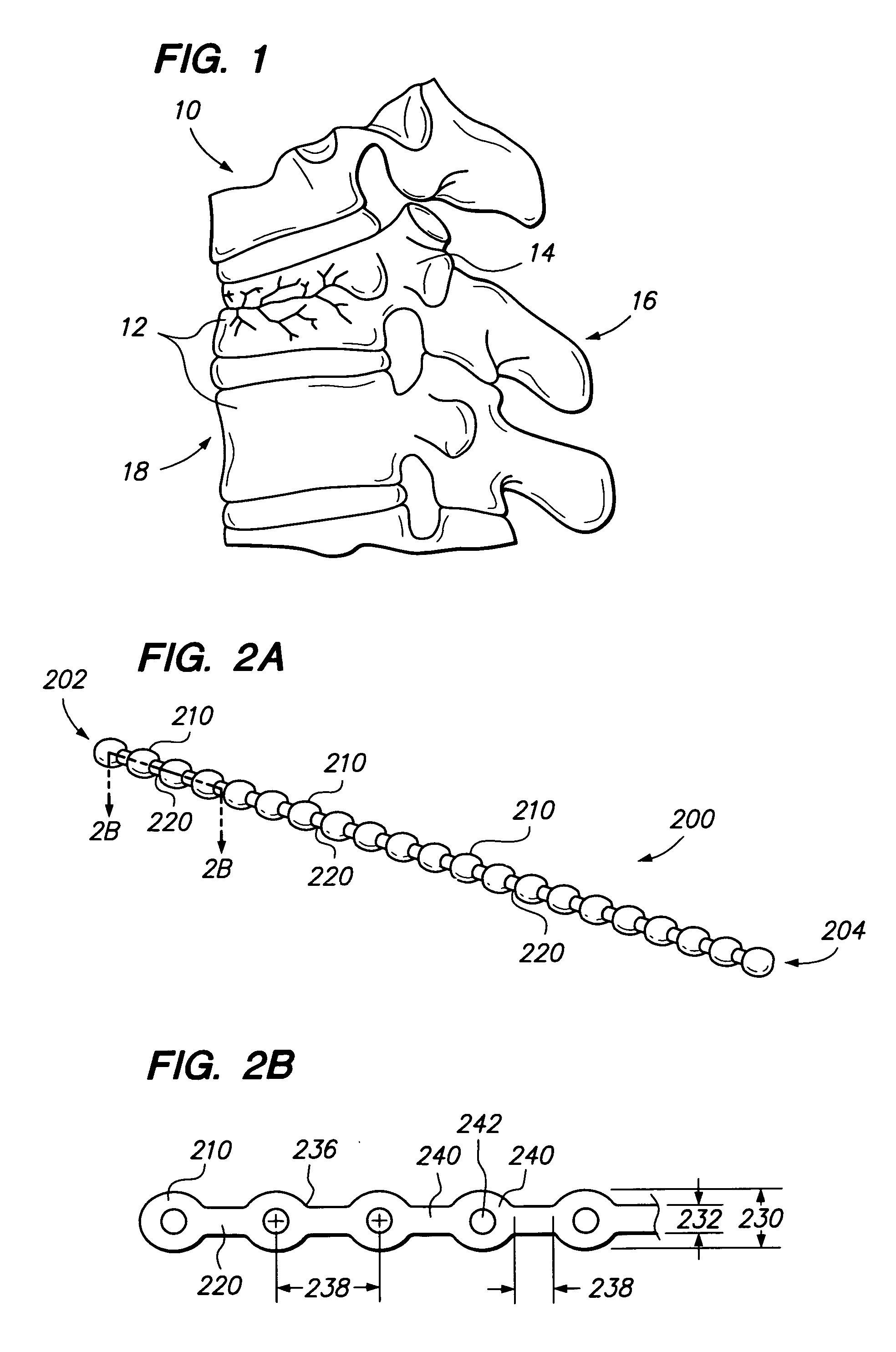
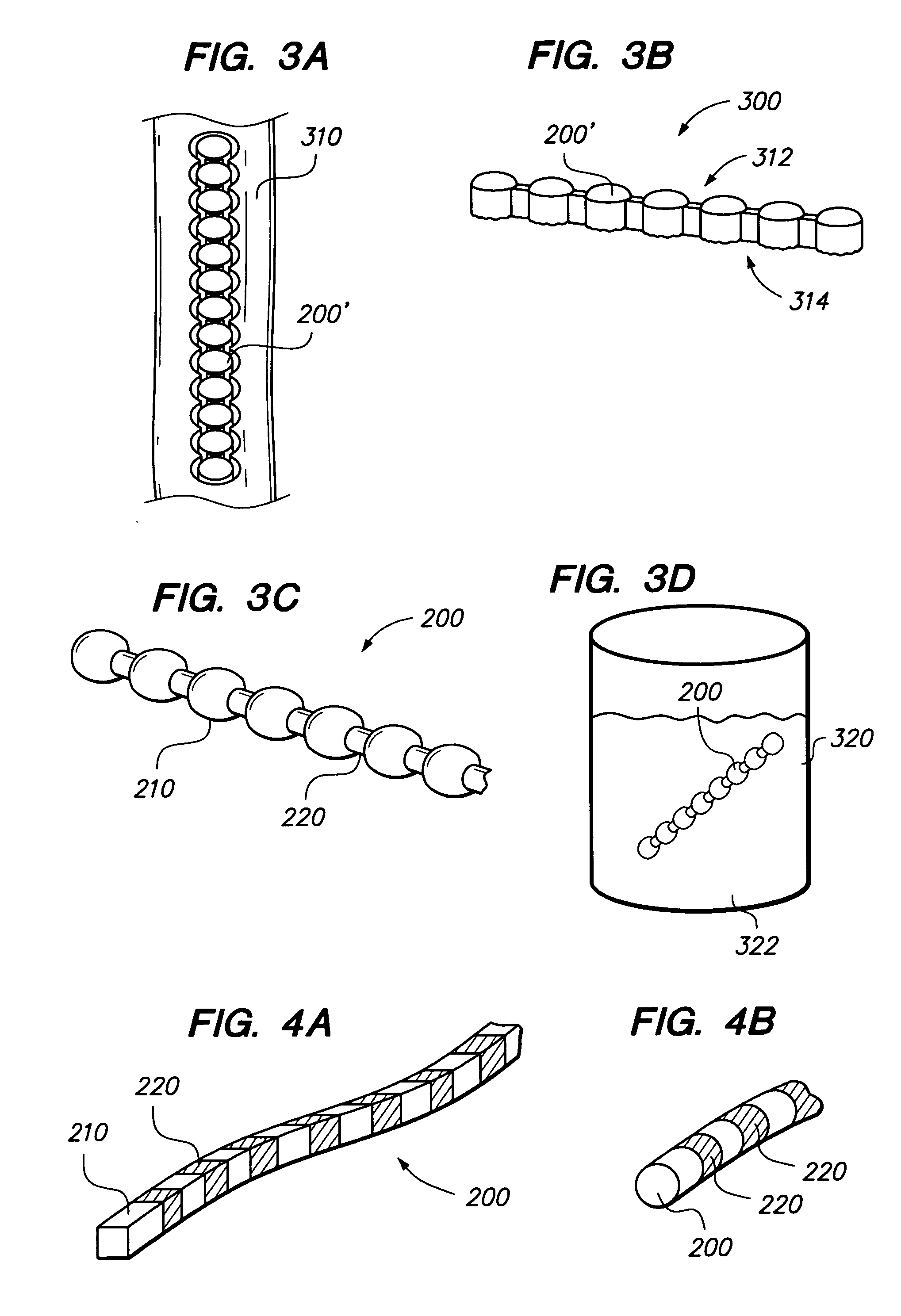
[0050] Referring to FIG. 2, a chain 200 (sometimes referred to as an elongated member) comprises one or more bodies 210 (sometimes referred to as beads). Chain 200 is preferably a monolithic chain, e.g., formed from a single, common material or type of material forming an integral structure. Bodies 210 are preferably substantially non-flexible, and may be solid, semi-solid, porous, non-porous, hollow, or any combination thereof. Chain 200 may also comprise one or more linking portions 220, also sometimes referred to as struts or links 220. Struts 220 may be disposed between each pair of adjacent bodies 210. Struts 220 are preferably substantially flexible or semiflexible, e.g. to allow for bending of the chain 200 between bodies 210.
[0051] Bodies 210 of chain 200 are preferably formed of bone, e.g., cortical bone, cancellous bone or both, but preferably cortical bone. In other embodiments, chain 200 may be comprised of any biocompatible material having desired characteristics, for ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| width | aaaaa | aaaaa |
| width | aaaaa | aaaaa |
| height | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com