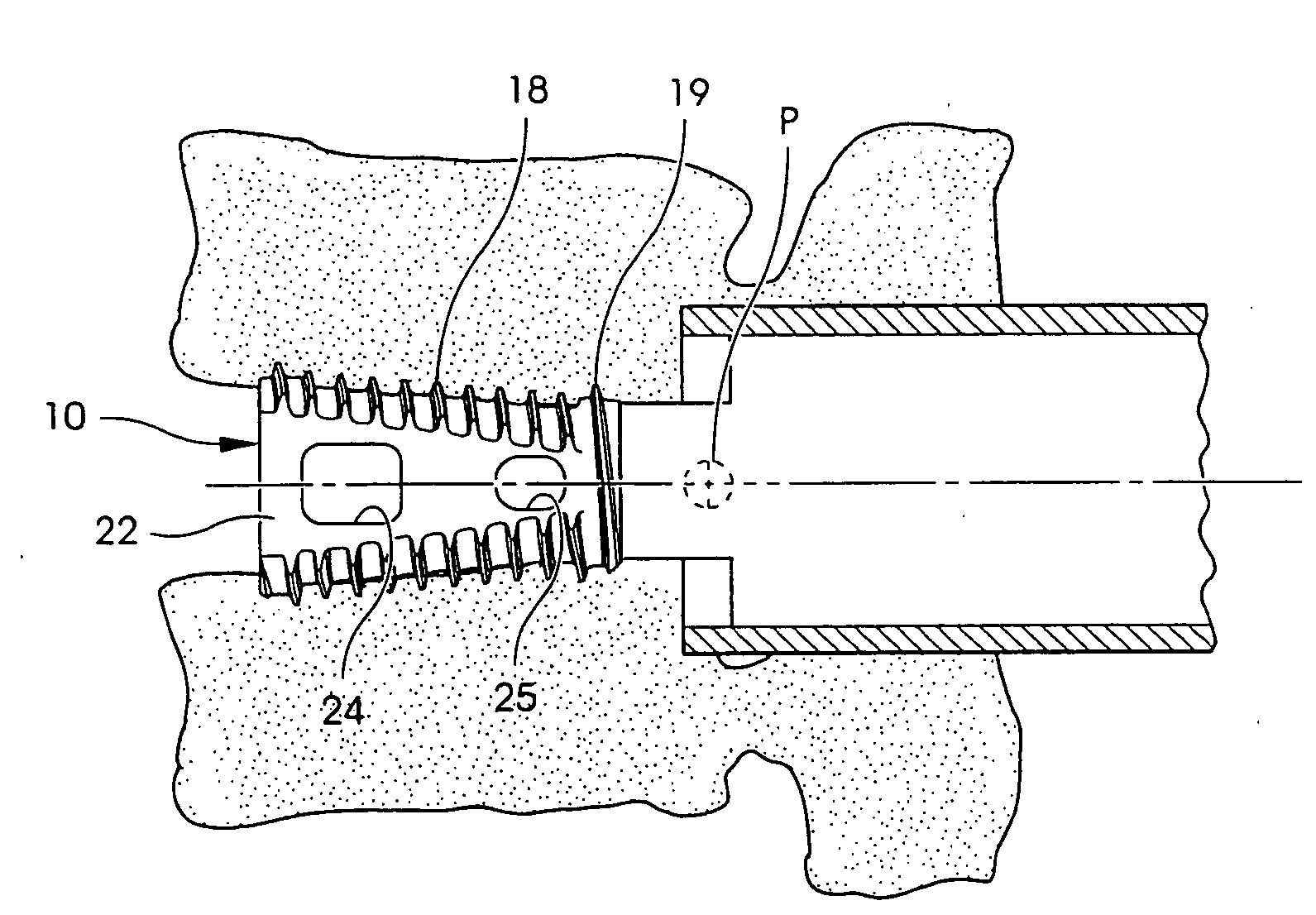
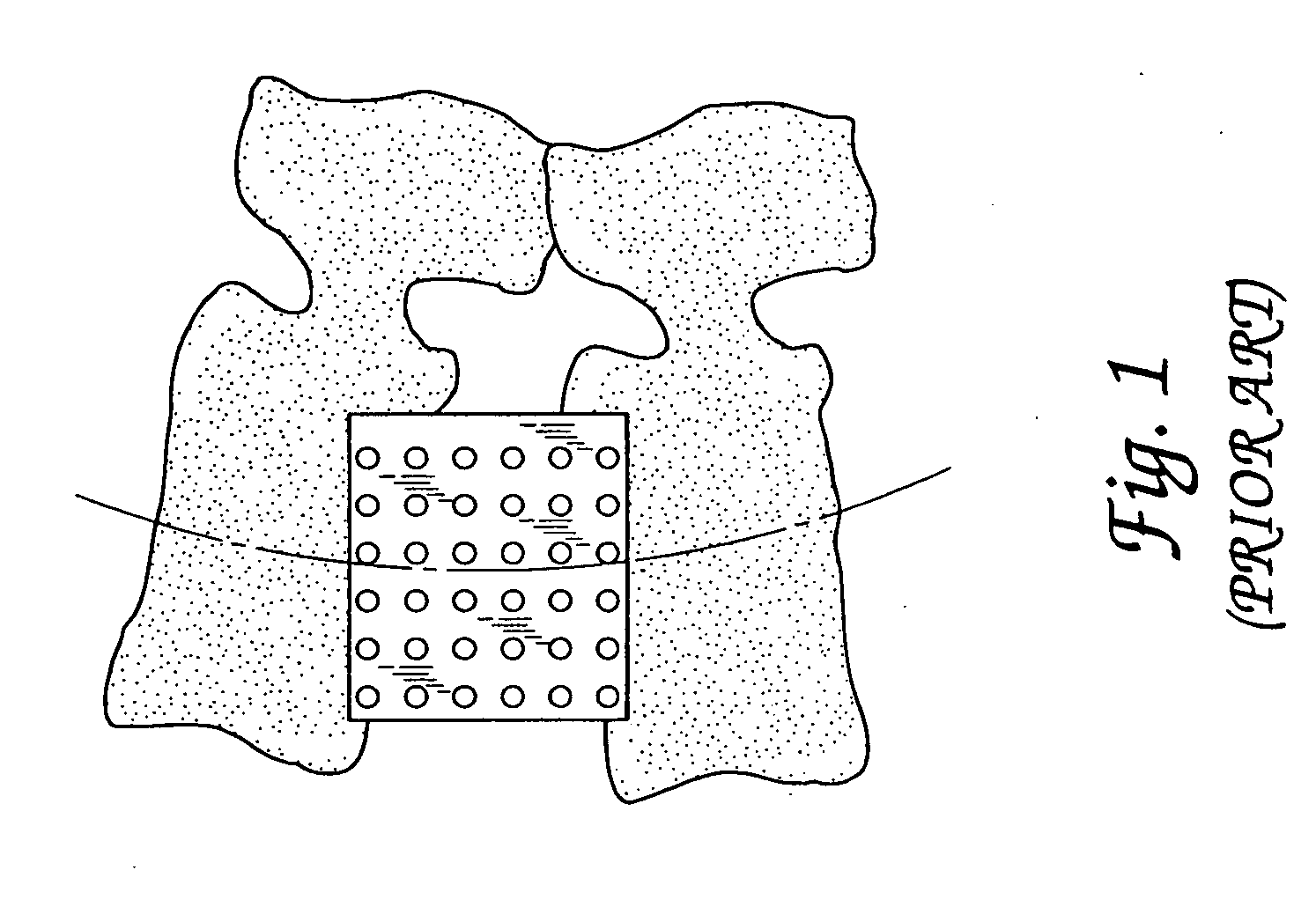
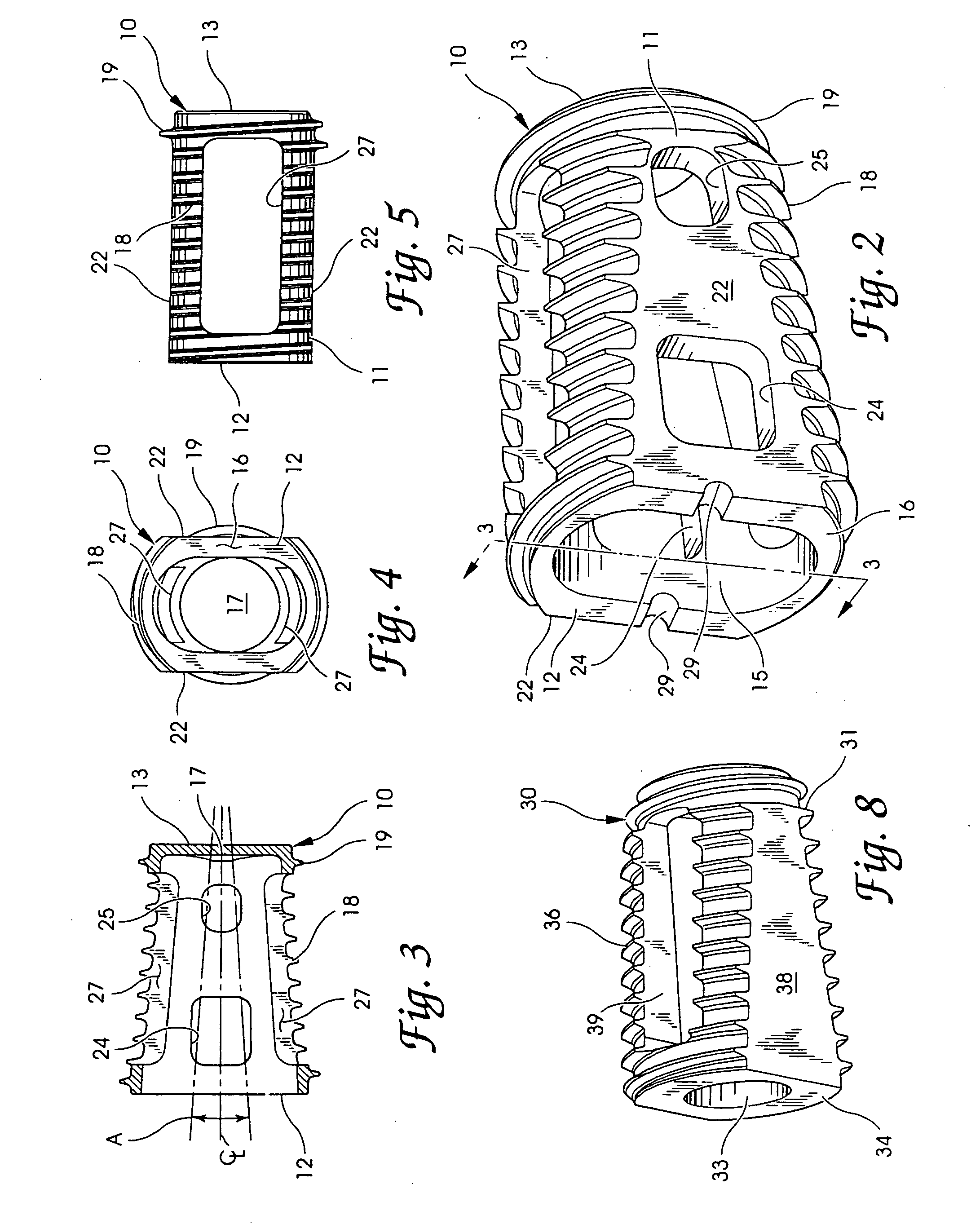
Spinal fusion implants and tools for insertion and revision
a technology of fusion implants and tools, applied in the field of artificial implants, can solve the problems of affecting the fusion effect, the surgical procedure necessary to implant rods or plates to stabilize the level during fusion, and the damage to the nerves extending along the spinal column. it can prevent the expulsion of osteogenic materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0108]Surgical Technique: Twenty-one mature female Alpine goats were used in this study. The goats weighed between 42 and 62 kilograms. All the goats underwent a surgical procedure under general endotracheal anesthesia using intravenous valium and ketamine for induction, and inhalation halothane for maintenance anesthesia. The anterior neck was prepped in a sterile fashion and a right anterolateral approach to the cervical spine was carried out through a longitudinal skin incision. The well developed longus coli muscle was incised in the midline, and the disc spaces at C2-C3, C3-C4, and C4-C5 exposed. Anterior cervical discectomies were carried out at each level by first excising the soft disc. An 8 mm distraction plug centered on a post was then tapped into the disc space providing distraction of the space. A working tube was then passed over the post and prongs at the end of the tube tapped into the vertebral bodies above and below the disc space. These prongs maintained distracti...
example 2
[0128]Design: Twelve mature female sheep underwent single level midlumbar interbody fusion. All surgical dissections were performed in an identical fashion. Following preparation of the anterior fusion sites the implants were inserted. Sheep were treated with a Threaded Interbody Fusion Device (TIBFD) containing rhBMP-2 carried on a type I fibrillar collagen (Helistat)(n=6) in a single cage, lateral orientation through a retroperitoneal approach. Previous limbs of the study (all n=6) included TIBFD with autogenous bone plugs, autogenous bone plugs alone, or sham (empty) fusion sites. The sheep were allowed to graze immediately post-operatively and no external immobilization was used. All animals were sacrificed six months following surgery. Fourteen additional cadaver sheep spines had been obtained to determine baseline intervertebral mechanical stiffness measures.
[0129]Materials: The interbody fusion cages developed and manufactured by Sofamor Danek, Inc., Memphis Tenn. were made o...
example 3
[0143]Open Porosity Polylactic Acid Polymer (OPLA) is provided in sterile packaged 12.0 mm×6.5 mm×30 mm strips (two strips per package). The pure OPLA is sterilized via gamma irradiation. The rhBMP-2 is provided in freeze-dried powder form and reconstituted intra-operatively in sterile water and supplemented with a buffer vehicle solution. The rhBMP-2 is introduced into the carrier material and the carrier is placed into the hollow interior of a metal fusion cage device. The device is then implanted at the fusion site.
PUM
| Property | Measurement | Unit |
|---|---|---|
| height | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| angle | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com