Sutureless implantable device and method for treatment of glaucoma
a sutureless, implantable technology, applied in the field of medical devices and methods, can solve the problems of vascular congestion, obstruction or blockage of aqueous humor drainage from the anterior chamber, and defect in the functional drainage system, and achieve the effect of minimal discomfort and easy fitting for patients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0034] The following detailed description, and the accompanying drawings to which it refers are provided for purposes of exemplifying and illustrating representative examples and embodiments of the invention only, and are not intended to limit the scope of the invention in any way. Indeed, no effort has been made to exhaustively illustrate and describe all possible embodiments and configurations in which the present invention may take physical form.
i. Construction and Configuration of the Fluid Shunting Device
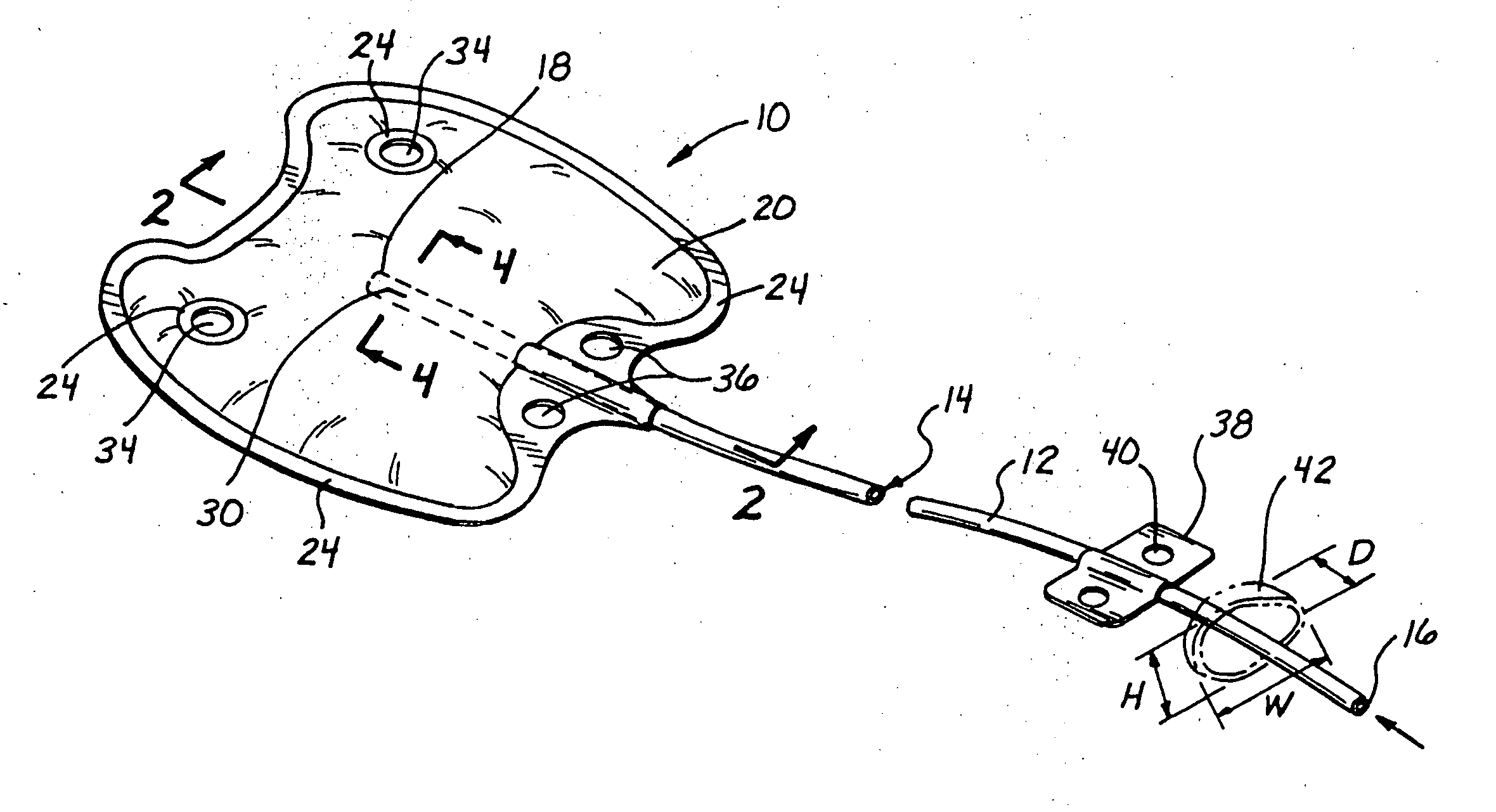
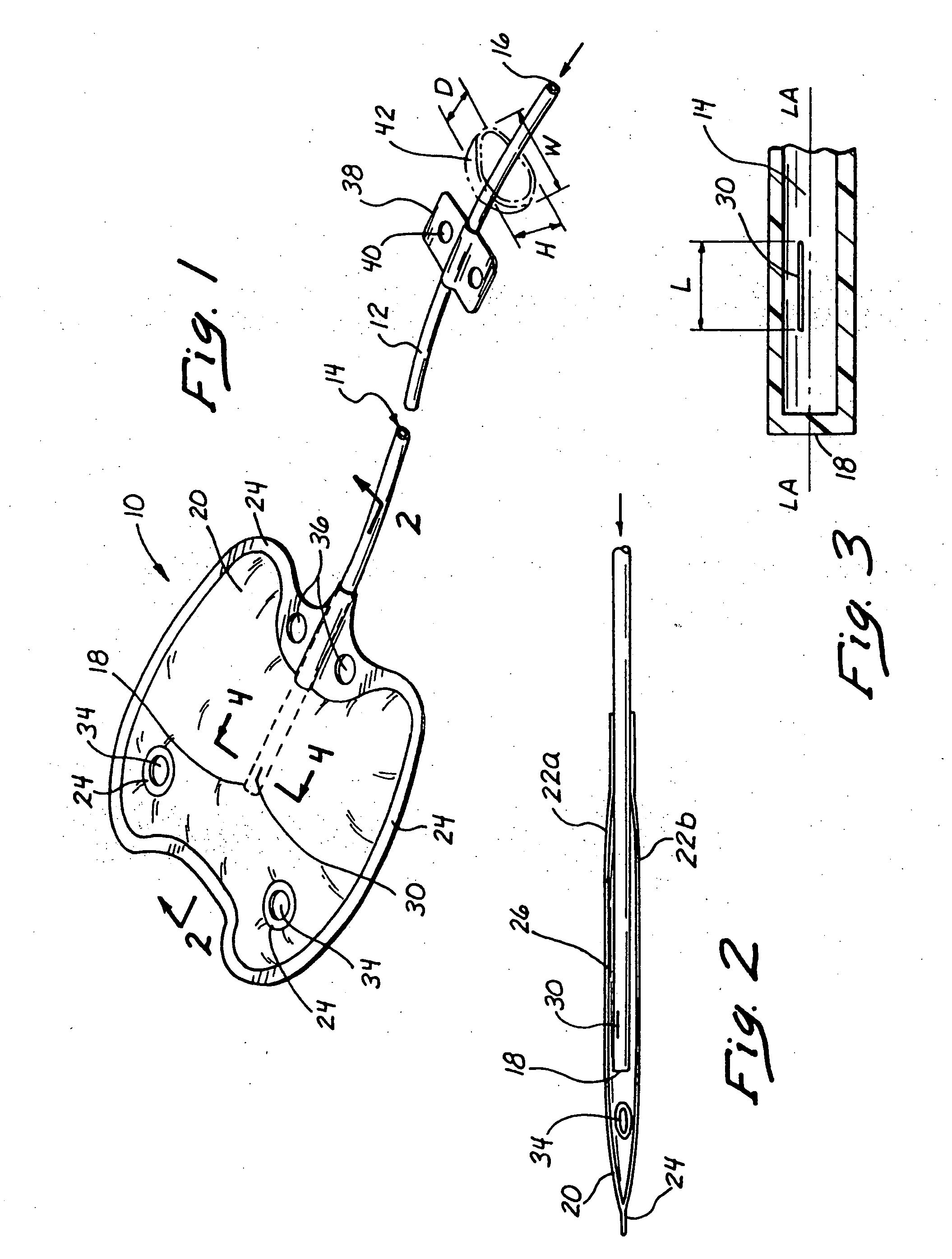
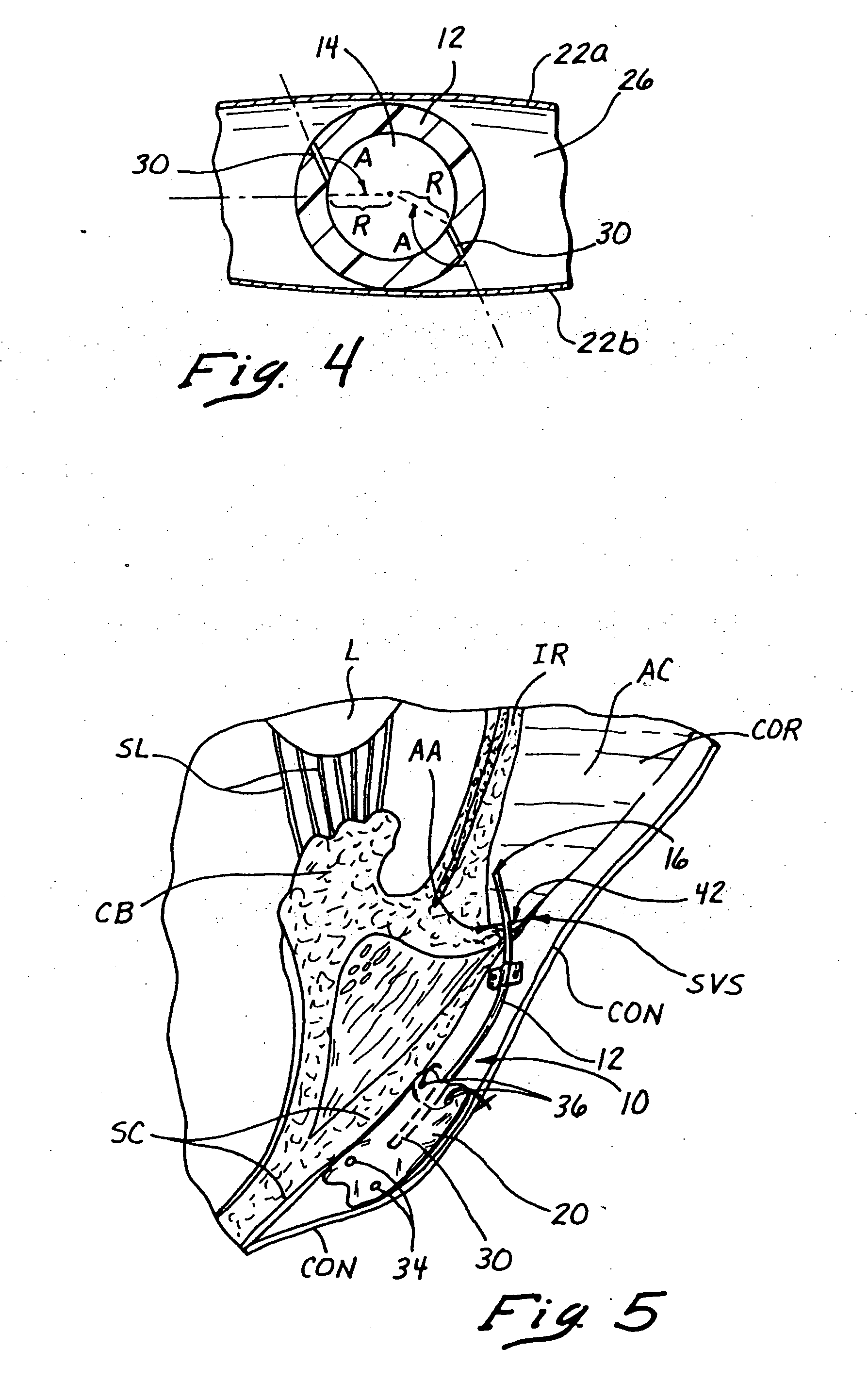
[0035] With reference to FIGS. 1-4, there is shown a first embodiment of an implantable fluid shunting device 10 comprising an elongate tube 12 having a lumen 14 extending longitudinally therethrough and a diffusion chamber 20 mounted on the proximal end thereof. The tube 12 has an open distal end 16, a closed proximal end 18 and a pressure openable aperture 30 which is located in a proximal portion PP of the tube 12 which extends into the interior of the diffusion chamber 2...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com