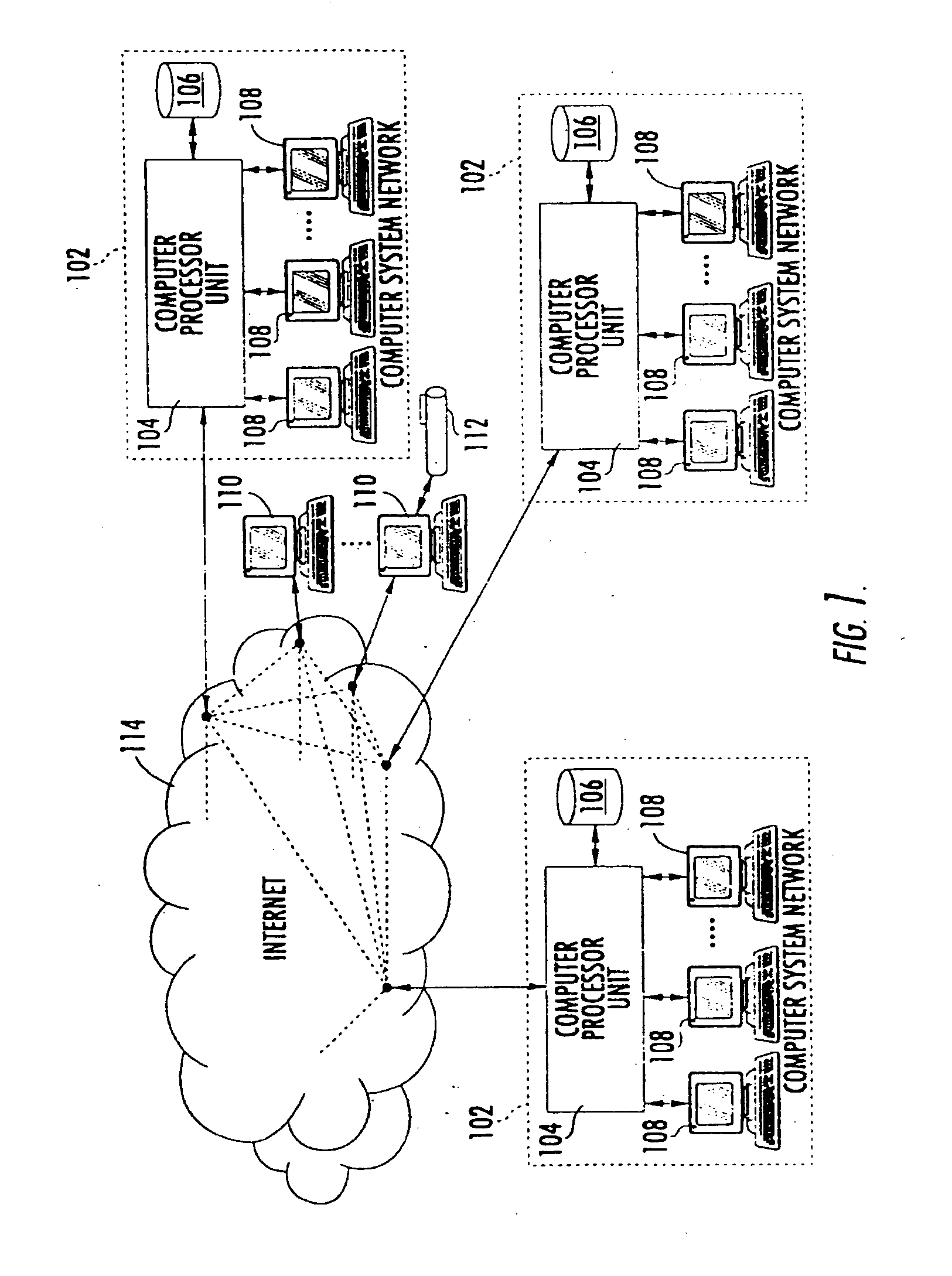
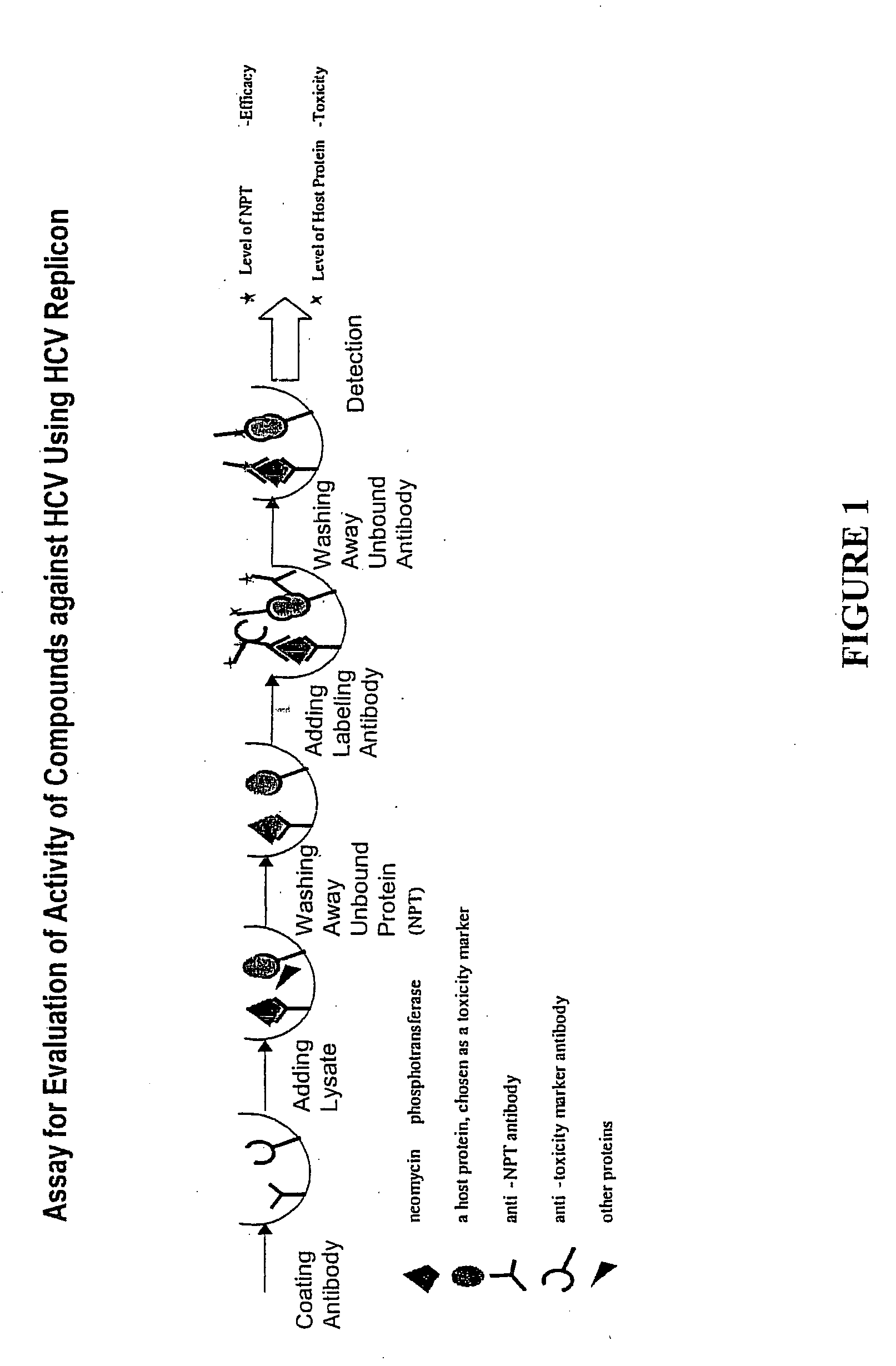
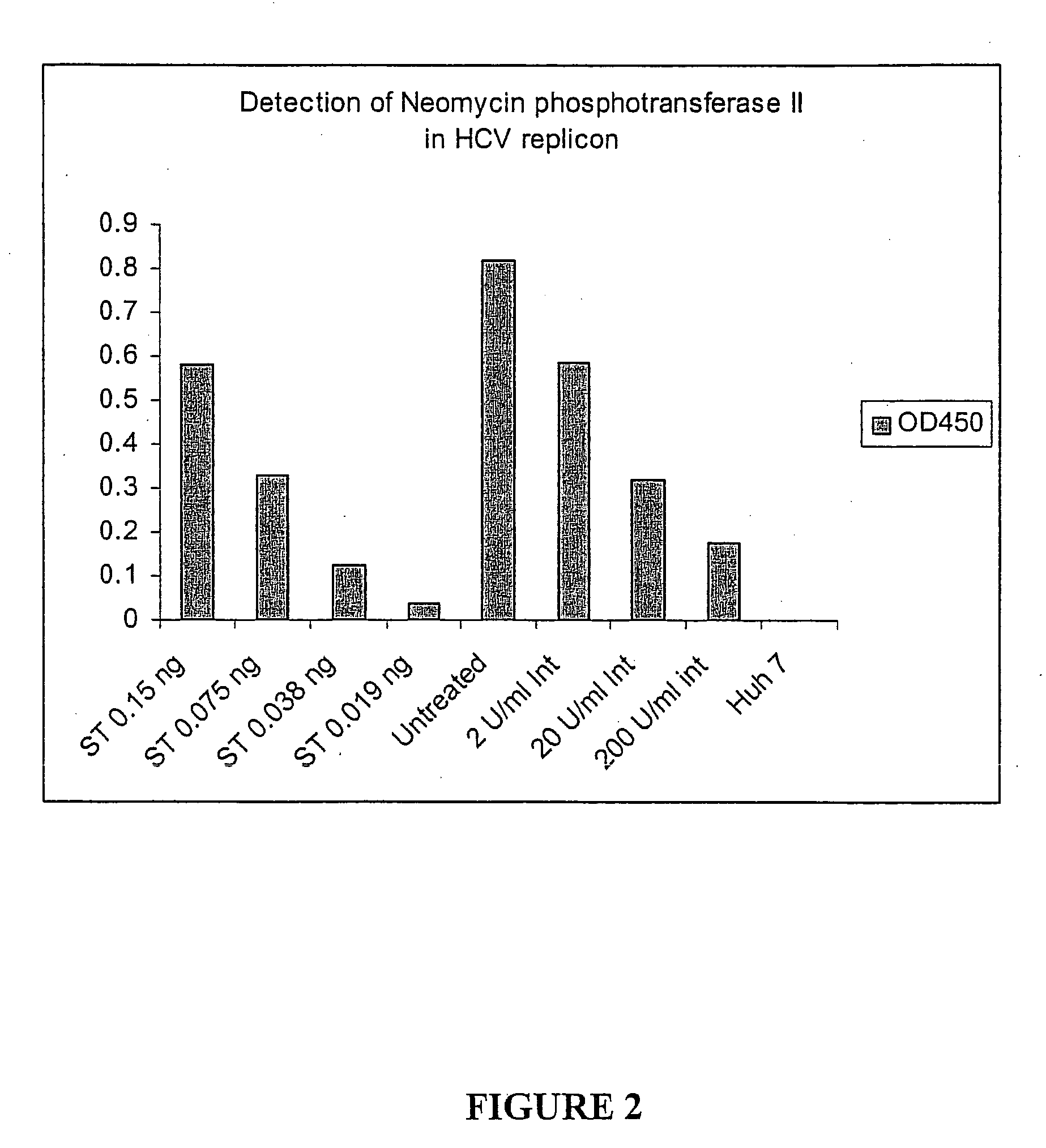
Patents
Literature
40 results about "Sign or Symptom" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Sign vs. symptom. A sign is the effect of a health problem that can be observed by someone else. A symptom is an effect noticed and experienced only by the person who has the condition. The key difference between signs and symptoms is who observes the effect.
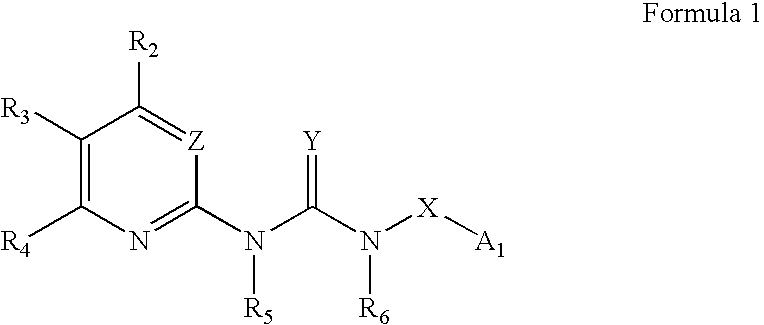
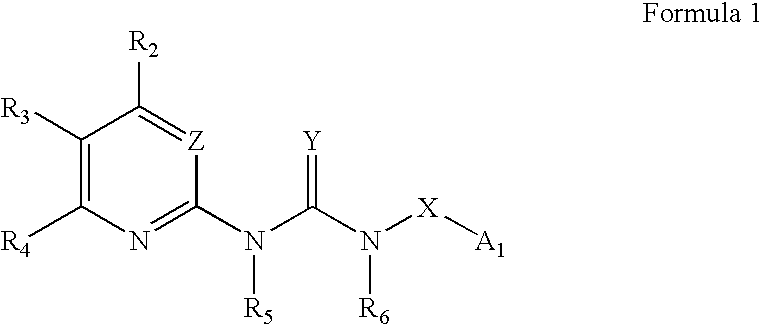
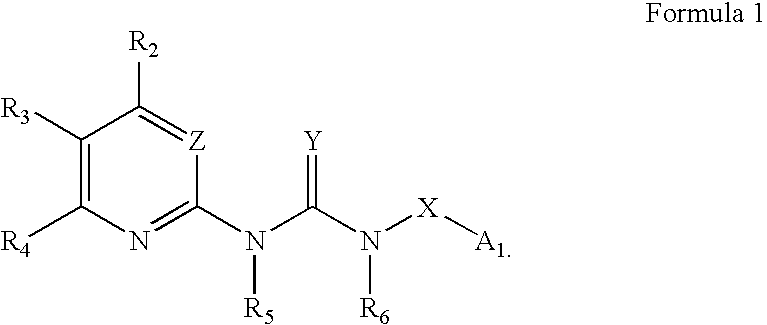
Certain heterocyclic substituted imidazo[1,2-A]pyrazin-8-ylamines and methods of inhibition of Bruton's tyrosine kinase by such compounds
ActiveUS7393848B2Prevent proliferationInhibit the activity of BtkBiocideOrganic chemistryActive agentBruton's tyrosine kinase
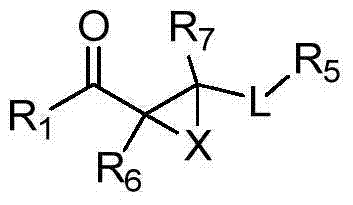
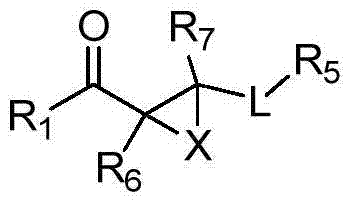
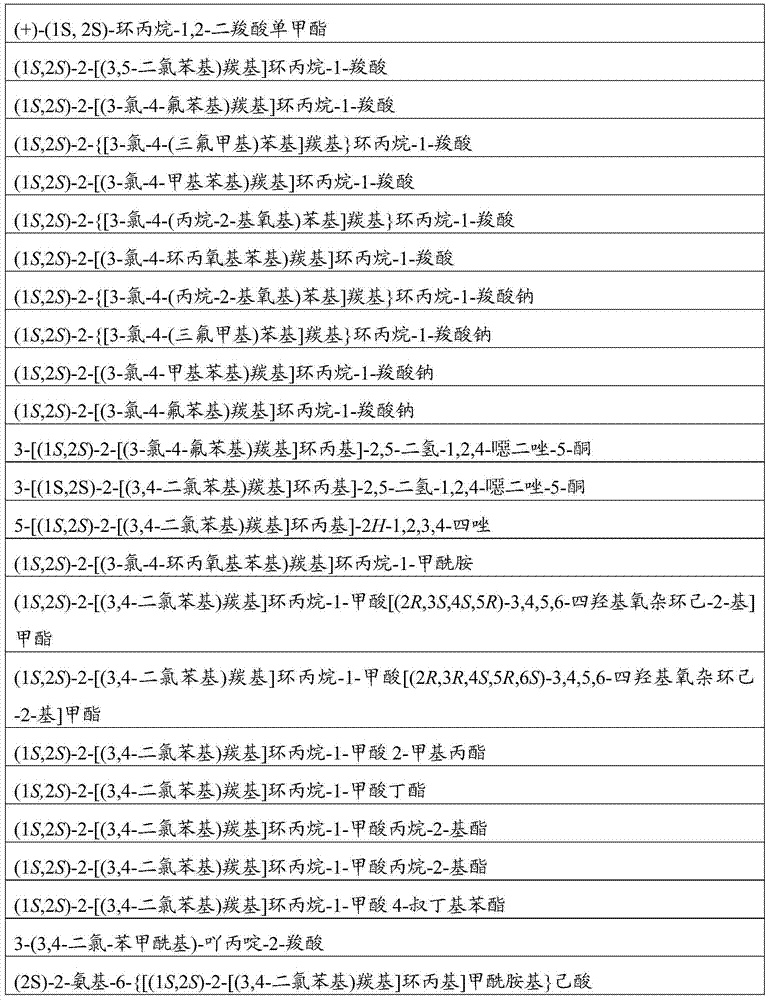
Compounds of Formula Iand all pharmaceutically acceptable forms thereof, are described herein.The variables R1, R2, R3, Z2, and Q, shown in Formula I are defined herein.Pharmaceutical compositions containing one or more compounds of Formula I, or a pharmaceutically acceptable form of such compounds, and one or more pharmaceutically acceptable carriers, excipients, or diluents are provided herein.Methods of treating patients suffering from certain diseases responsive to inhibition of tyrosine kinase activity are also given. In certain embodiments the diseases are responsive to inhibition of Btk activity and / or B-cell proliferation. Such methods comprise administering to such patients an amount of a compound of Formula I effective to reduce signs or symptoms of the disease. These diseases include cancer, an autoimmune and / or inflammatory disease, or an acute inflammatory reaction. Thus methods of treatment include administering a sufficient amount of a compound or salt as provided herein to decrease the symptoms or slow the progression of these diseases.Other embodiments include methods of treating other animals, including livestock and domesticated companion animals, suffering from a disease responsive to inhibition of kinase activity.Methods of treatment include administering a compound of Formula I as a single active agent or administering a compound of Formula I in combination with one or more other therapeutic agent.A method for determining the presence of Btk in a sample, comprising contacting the sample with a compound or form thereof of Formula I under conditions that permit detection of Btk activity, detecting a level of Btk activity in the sample, and therefrom determining the presence or absence of Btk in the sample.
Owner:GILEAD CONNENTICUT INC
Food Compositions and Methods of Treating Periodontal Disease
The invention provides a method of alleviating a sign or symptom of periodontal disease in a subject, by administering to the subject a composition containing a natural compound which inhibits matrix metalloproteinase activity and interleukin-1 activity.
Owner:INTERLEUKIN GENETICS
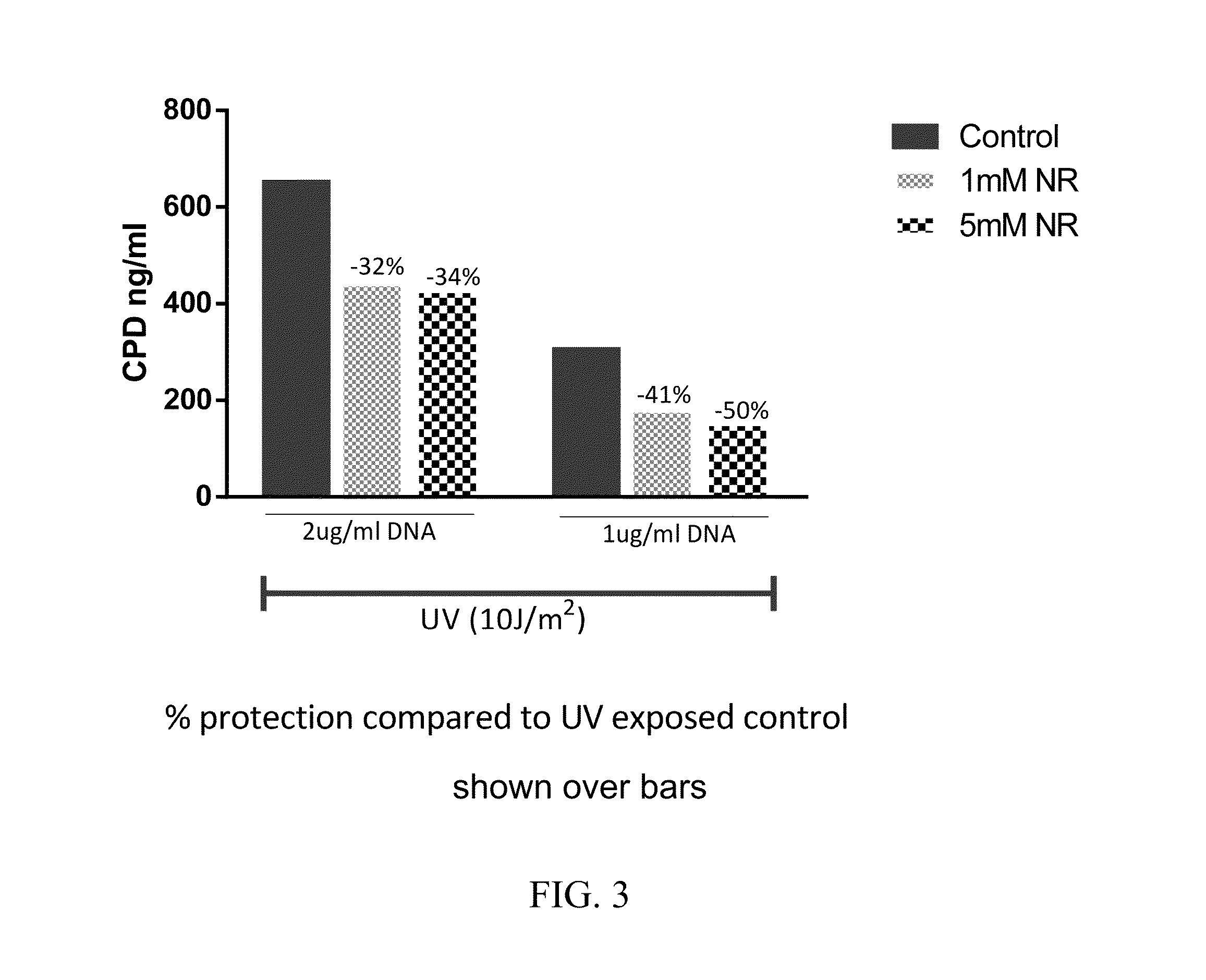
Nicotinamide riboside compositions for topical use in treating skin conditions
ActiveUS20160250241A1Increased motilityIncreased proliferationCosmetic preparationsOrganic active ingredientsNicotinamide ribosideTopical treatment
Compositions containing nicotinamide riboside (NR) are provided. NR containing compositions are used in the care or treatment of skin and skin conditions. In some embodiments, the invention relates to pharmaceutical compositions and cosmetic compositions containing nicotinamide riboside. In further embodiments, the invention relates to methods of using nicotinamide riboside to promote the increase of intracellular levels of nicotinamide adenine dinucleotide (NAD+) in cells and tissues for improving cell and tissue survival. A method of treating signs or symptoms of aging or skin wrinkles in an individual is provided, comprising topically administering to the individual in need of such treatment an effective amount of the compound nicotinamide riboside, or salts thereof.
Owner:CHROMADEX
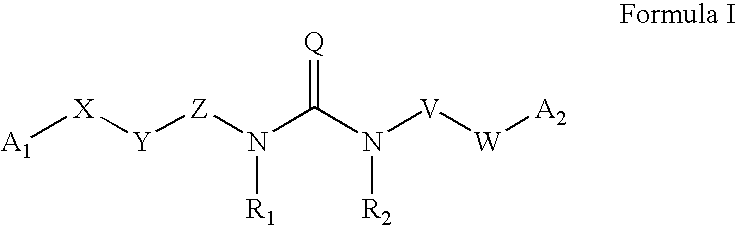
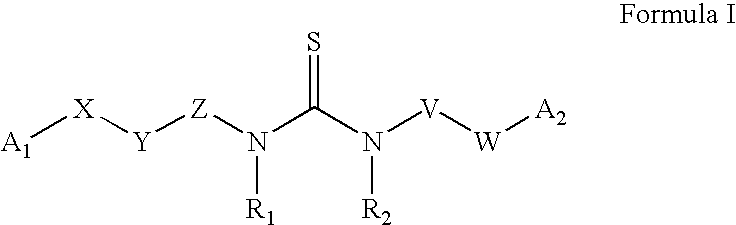
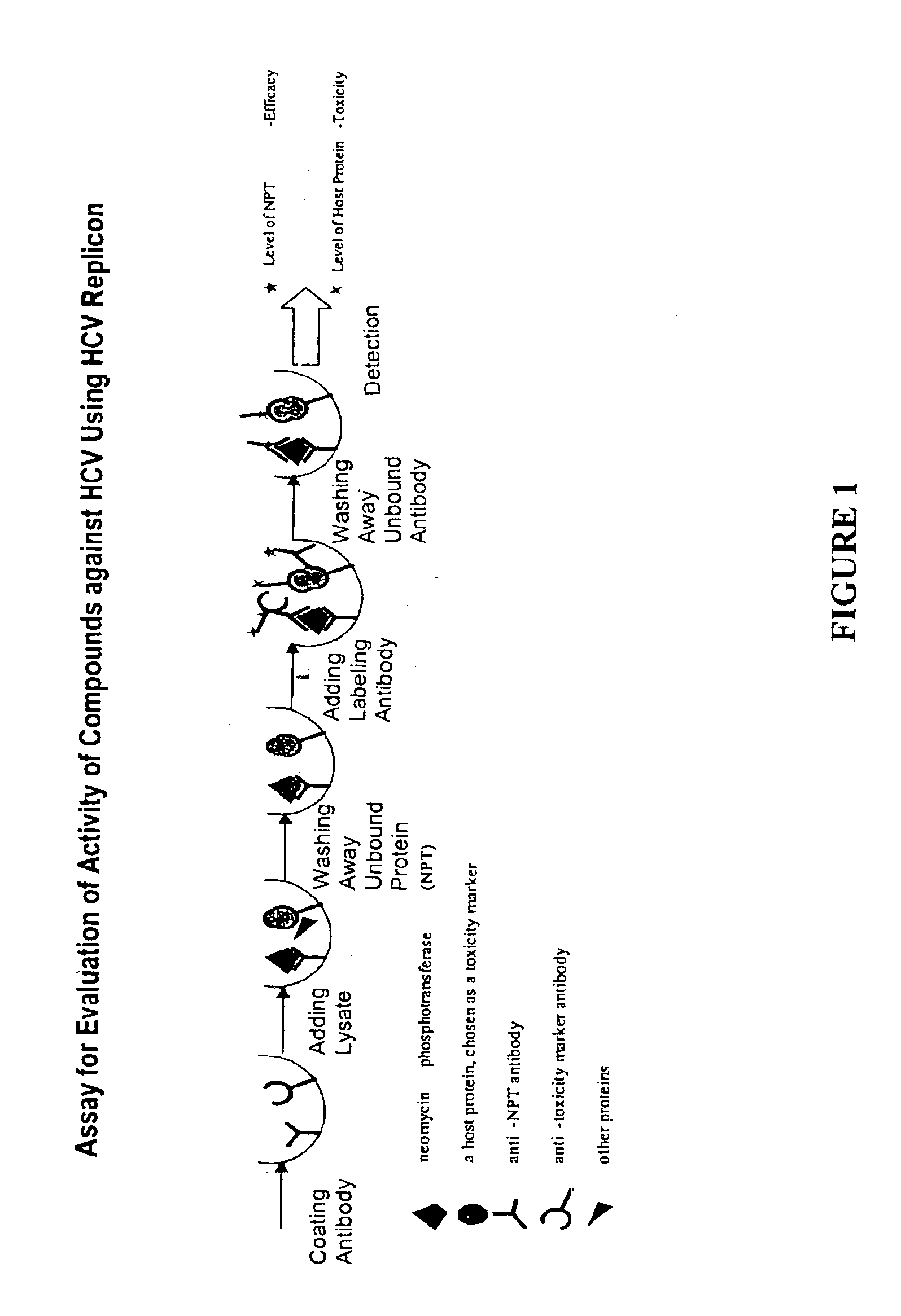
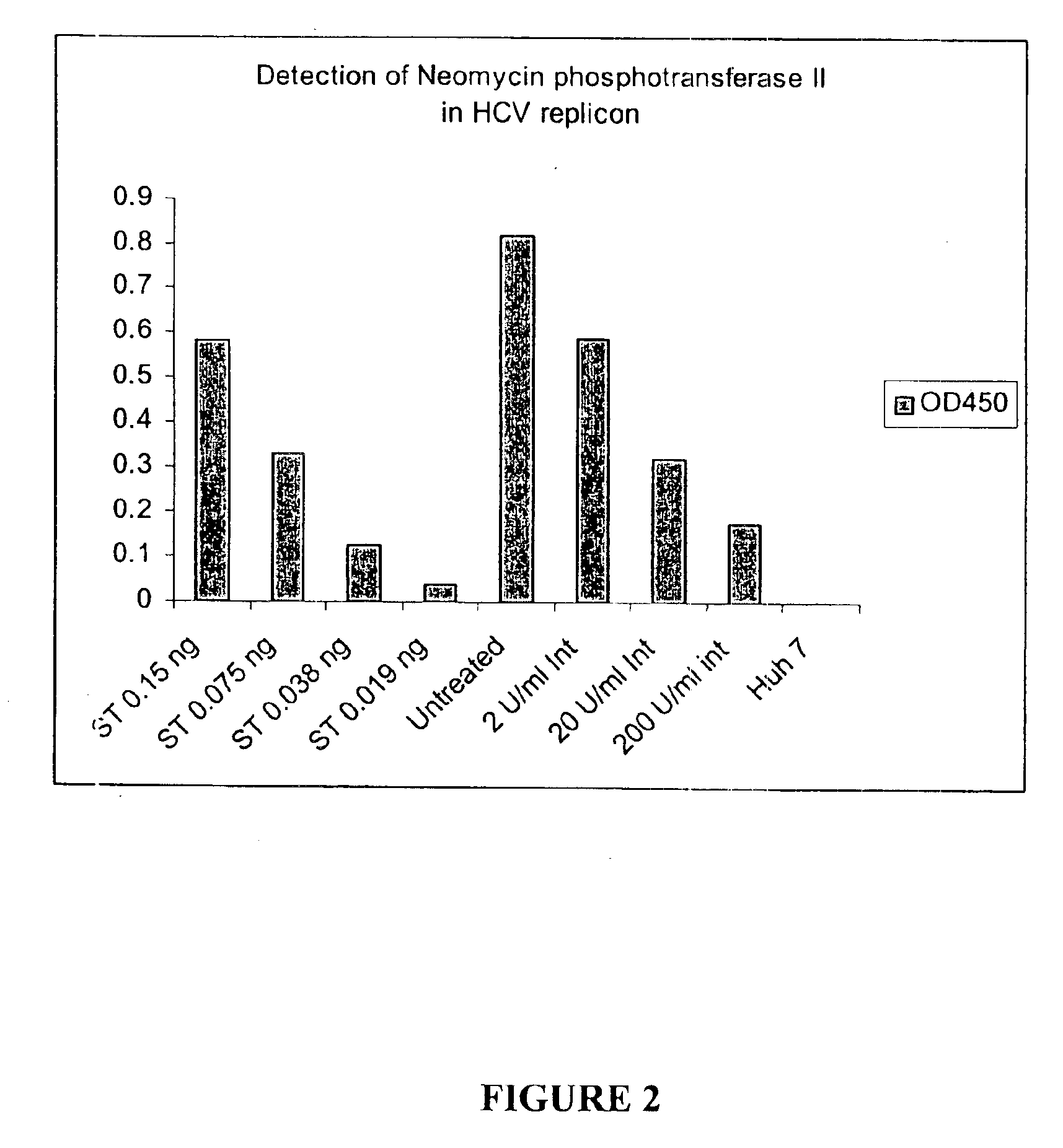
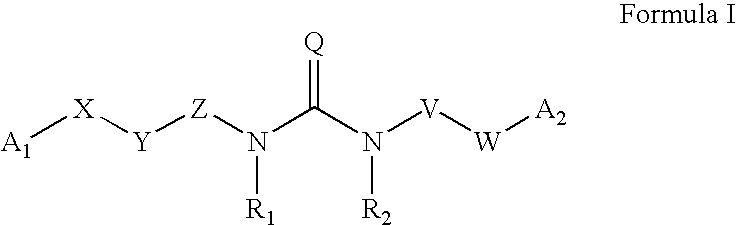
Substituted aryl acylthioureas and related compounds; inhibitors of viral replication
The invention provides compounds and pharmaceutically acceptable salts of Formula I wherein the variables A1, A2, R1, R2, V, W, X, Y, and Z are defined herein. Certain compounds of Formula I described herein which possess potent antiviral activity. The invention particularly provides compounds of Formula I that are potent and / or selective inhibitors of Hepatitis C virus replication. The invention also provides pharmaceutical compositions containing one or more compound of Formula I, or a salt, solvate, or acylated prodrug of such compounds, and one or more pharmaceutically acceptable carriers, excipients, or diluents. The invention further comprises methods of treating patients suffering from certain infectious diseases by administering to such patients an amount of a compound of Formula I effective to reduce signs or symptoms of the disease or disorder. These infectious diseases include viral infections, particularly HCV infections. The invention is particularly includes methods of treating human patients suffering from an infectious disease, but also encompasses methods of treating other animals, including livestock and domesticated companion animals, suffering from an infectious disease. Methods of treatment include administering a compound of Formula I as a single active agent or administering a compound of Formula I in combination with on or more other therapeutic agent.
Owner:ACHILLION PHARMA INC
Substituted aryl thioureas and related compounds; inhibitors of viral replication
The invention provides compounds and pharmaceutically acceptable salts of Formula I wherein the variables A1, A2, R1, R2, V, W, X, Y, and Z are defined herein. Certain compounds of Formula I described herein which possess potent antiviral activity. The invention particularly provides compounds of Formula I that are potent and / or selective inhibitors of Hepatitis C virus replication. The invention also provides pharmaceutical compositions containing one or more compound of Formula I, or a salt, solvate, or acylated prodrug of such compounds, and one or more pharmaceutically acceptable carriers, excipients, or diluents.The invention further comprises methods of treating patients suffering from certain infectious diseases by administering to such patients an amount of a compound of Formula I effective to reduce signs or symptoms of the disease or disorder. These infectious diseases include viral infections, particularly HCV infections. The invention is particularly includes methods of treating human patients suffering from an infectious disease, but also encompasses methods of treating other animals, including livestock and domesticated companion animals, suffering from an infectious disease.Methods of treatment include administering a compound of Formula I as a single active agent or administering a compound of Formula I in combination with on or more other therapeutic agent.
Owner:ACHILLION PHARMA INC
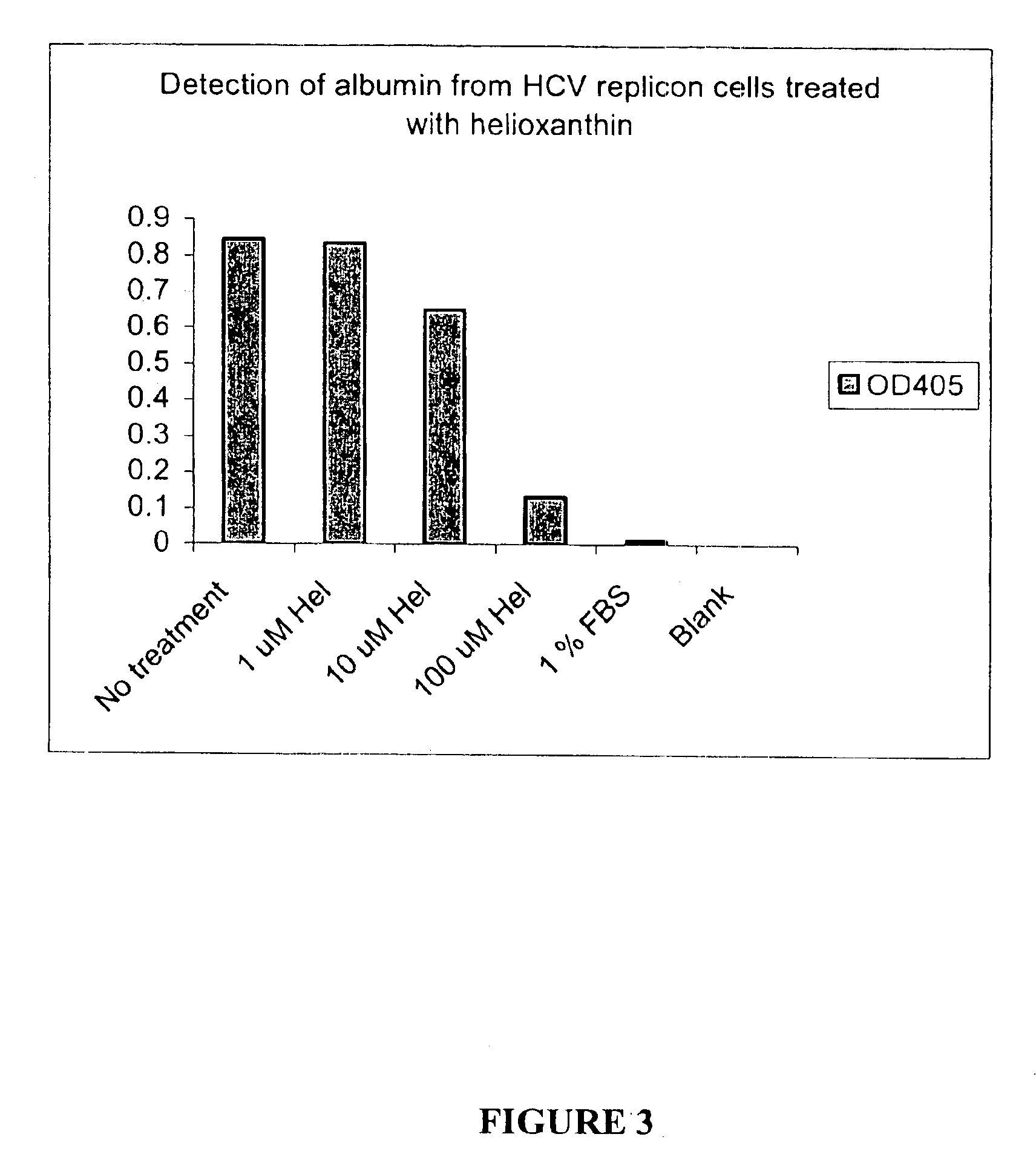
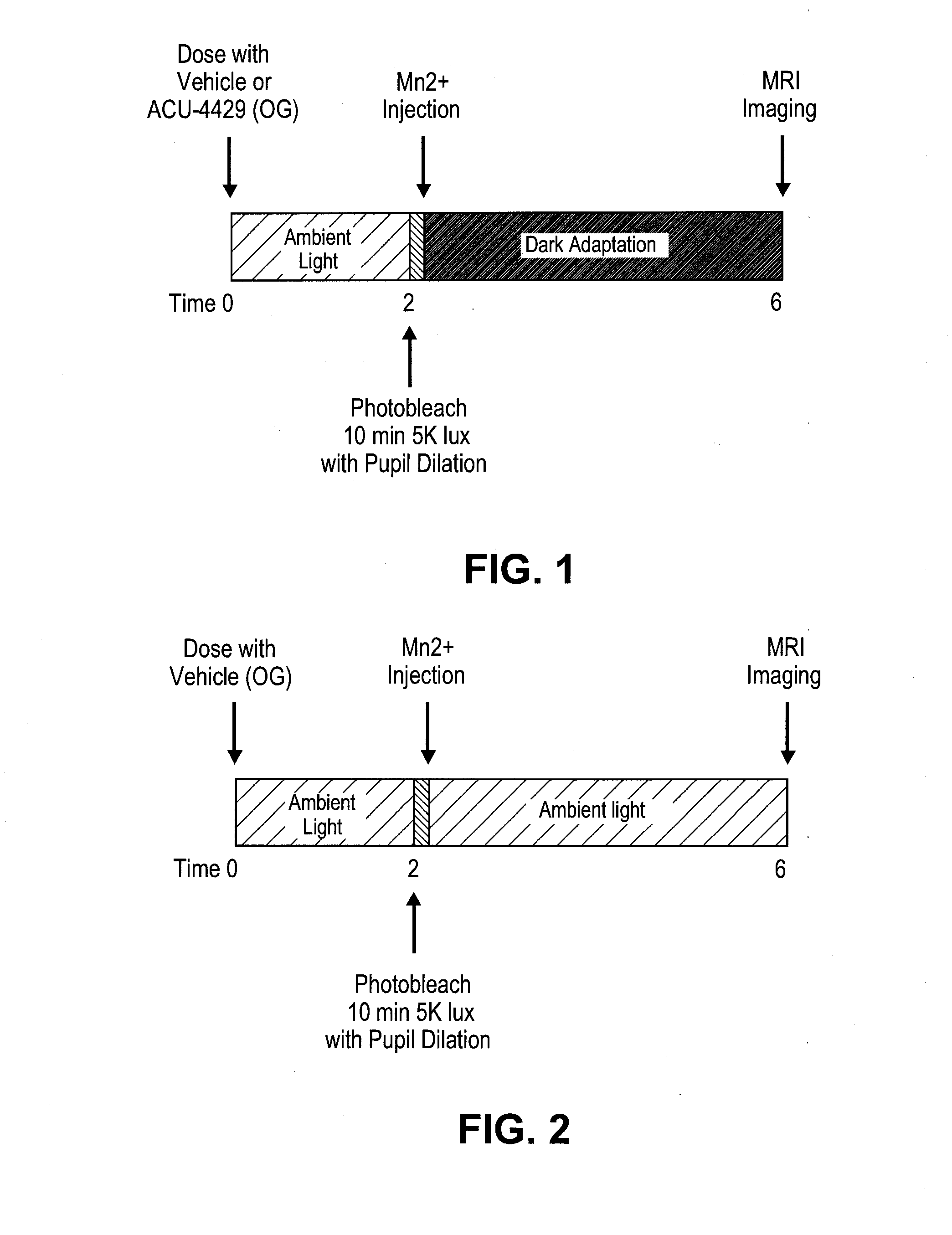
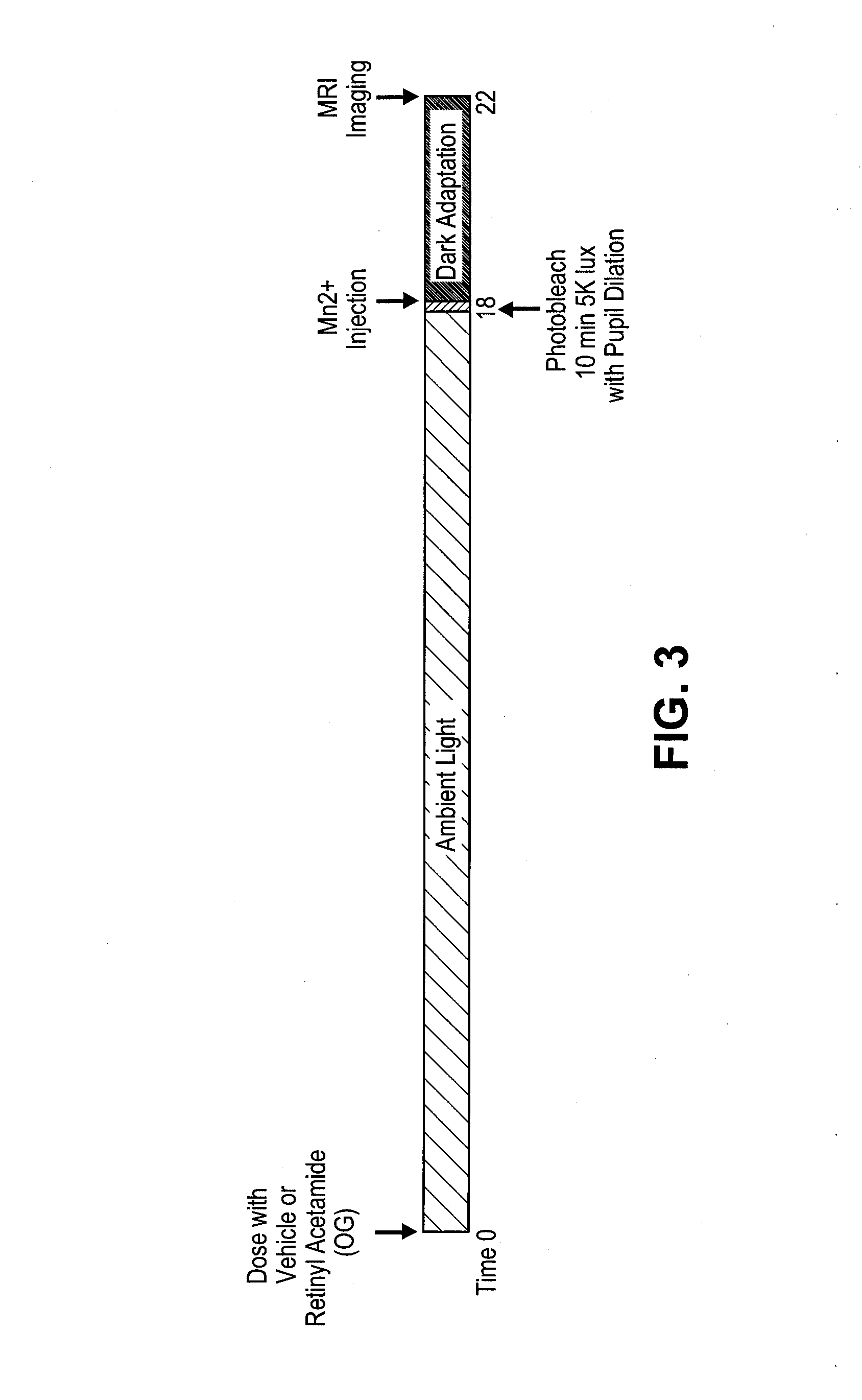
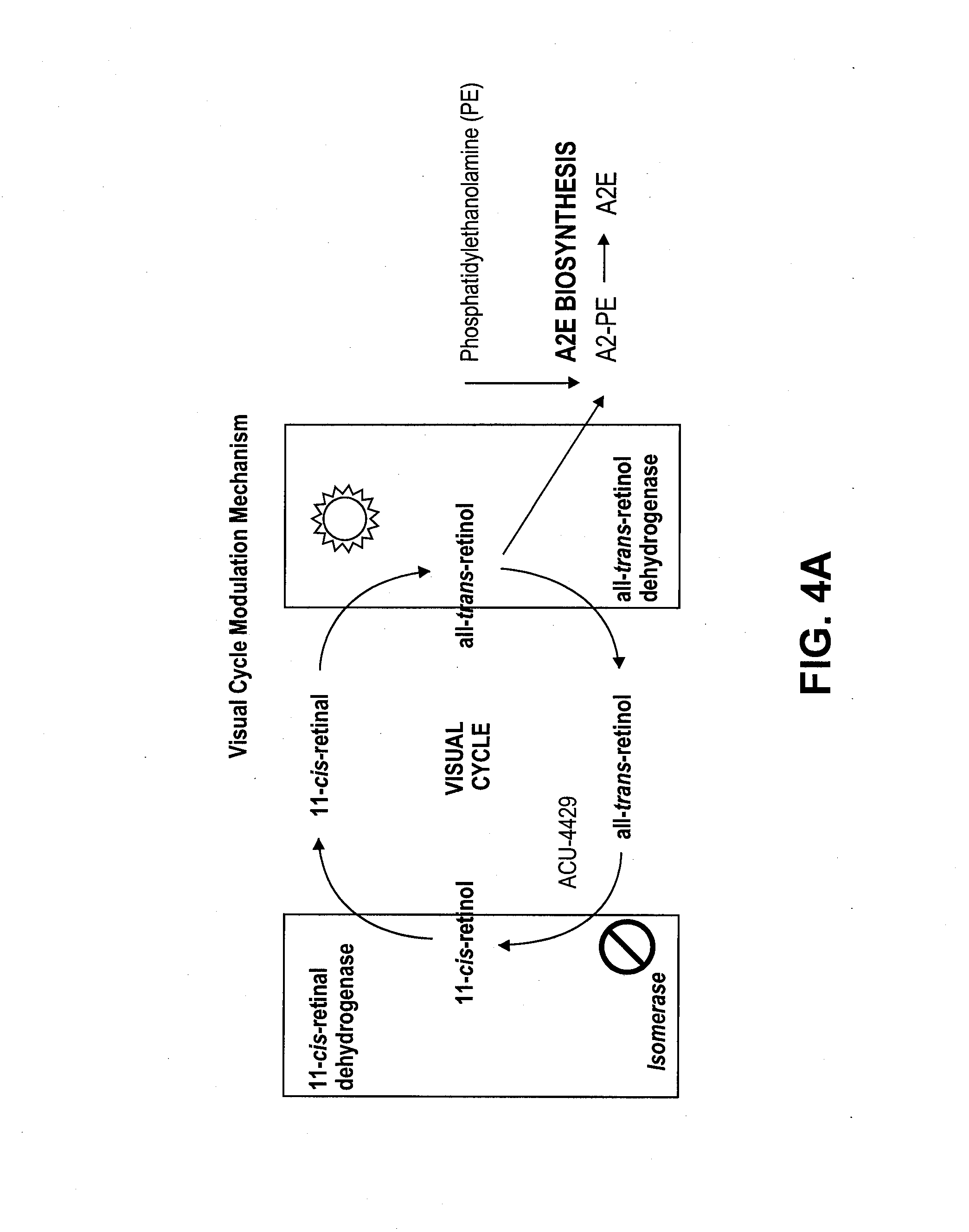
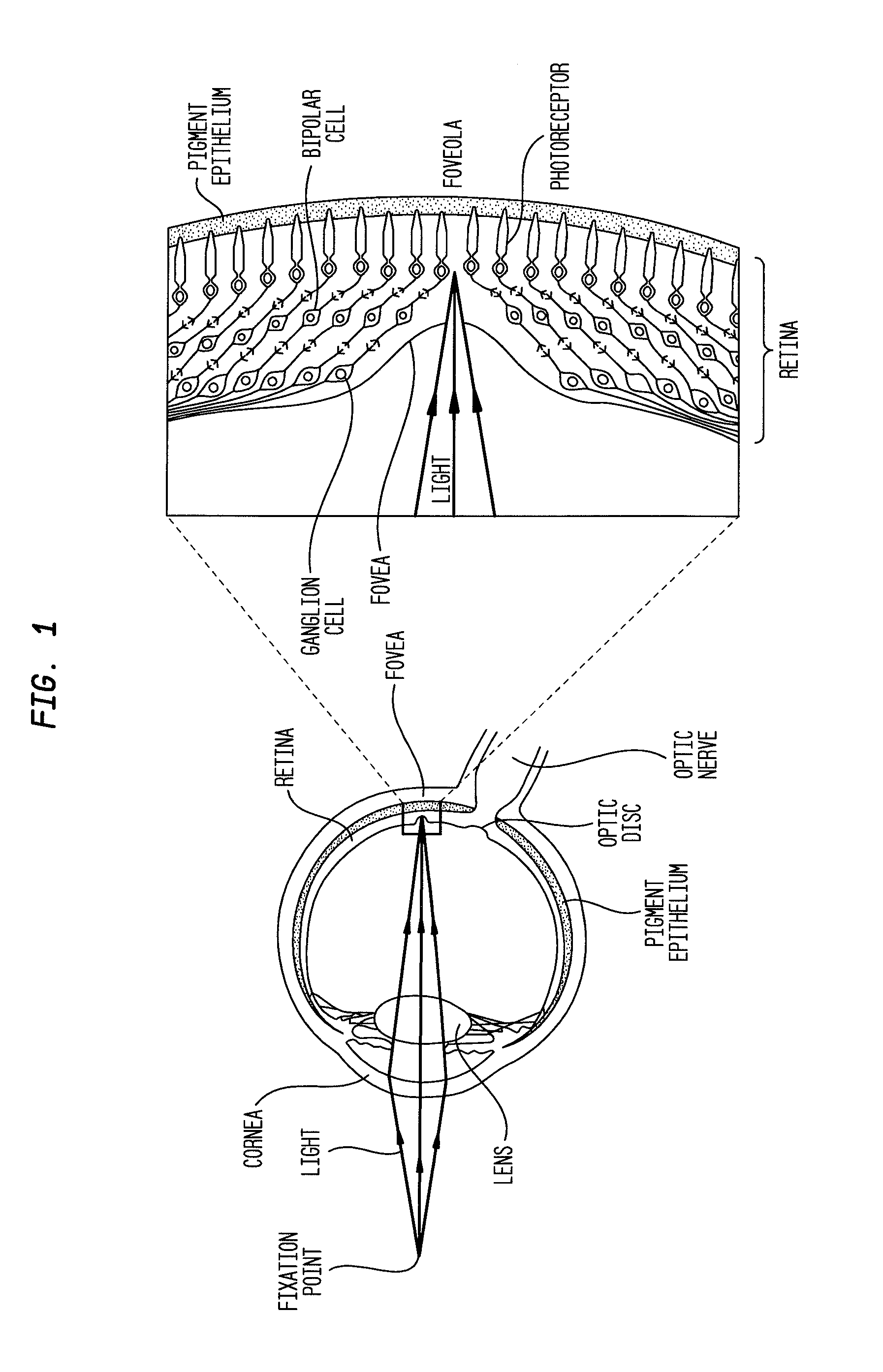
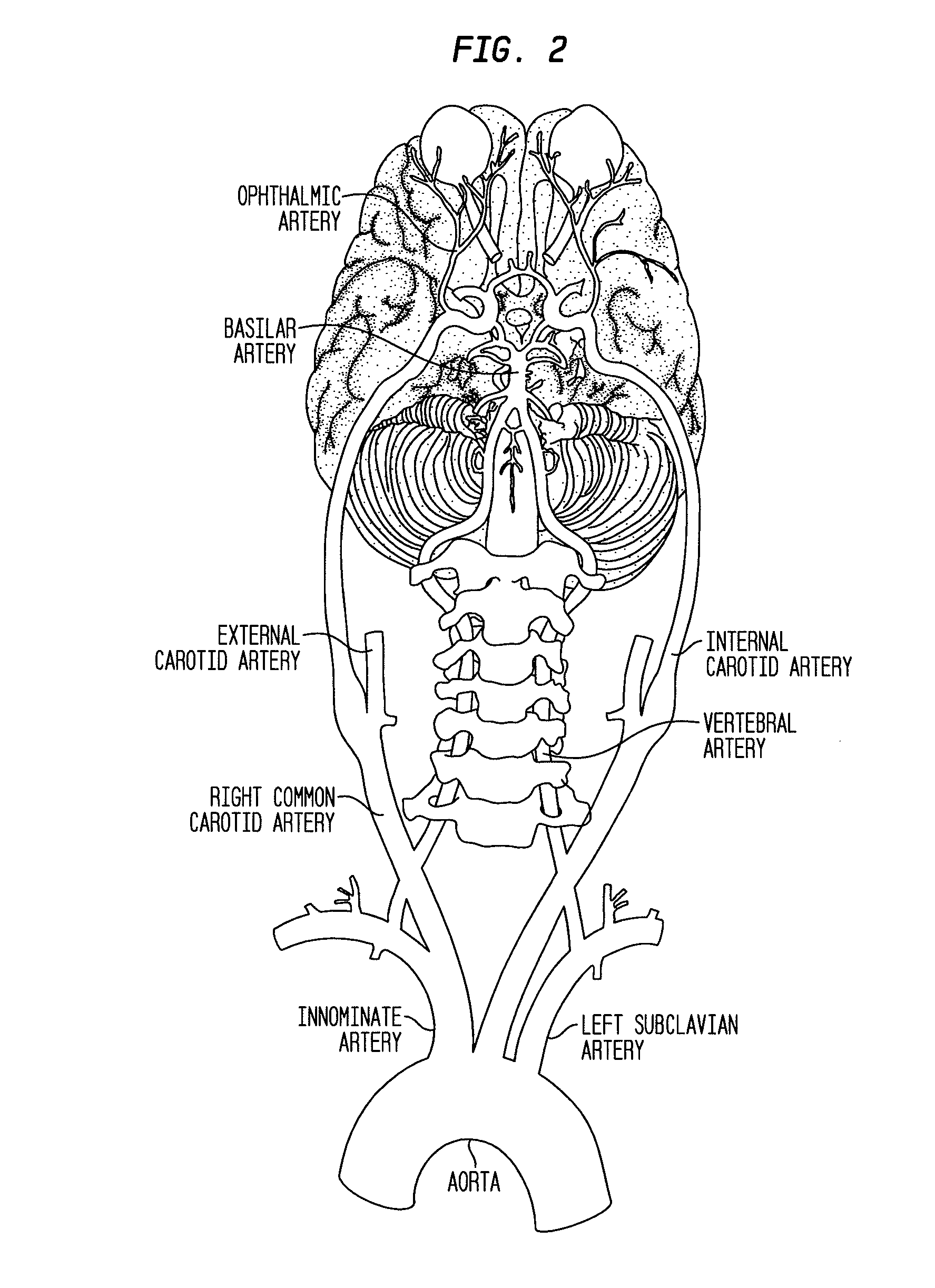
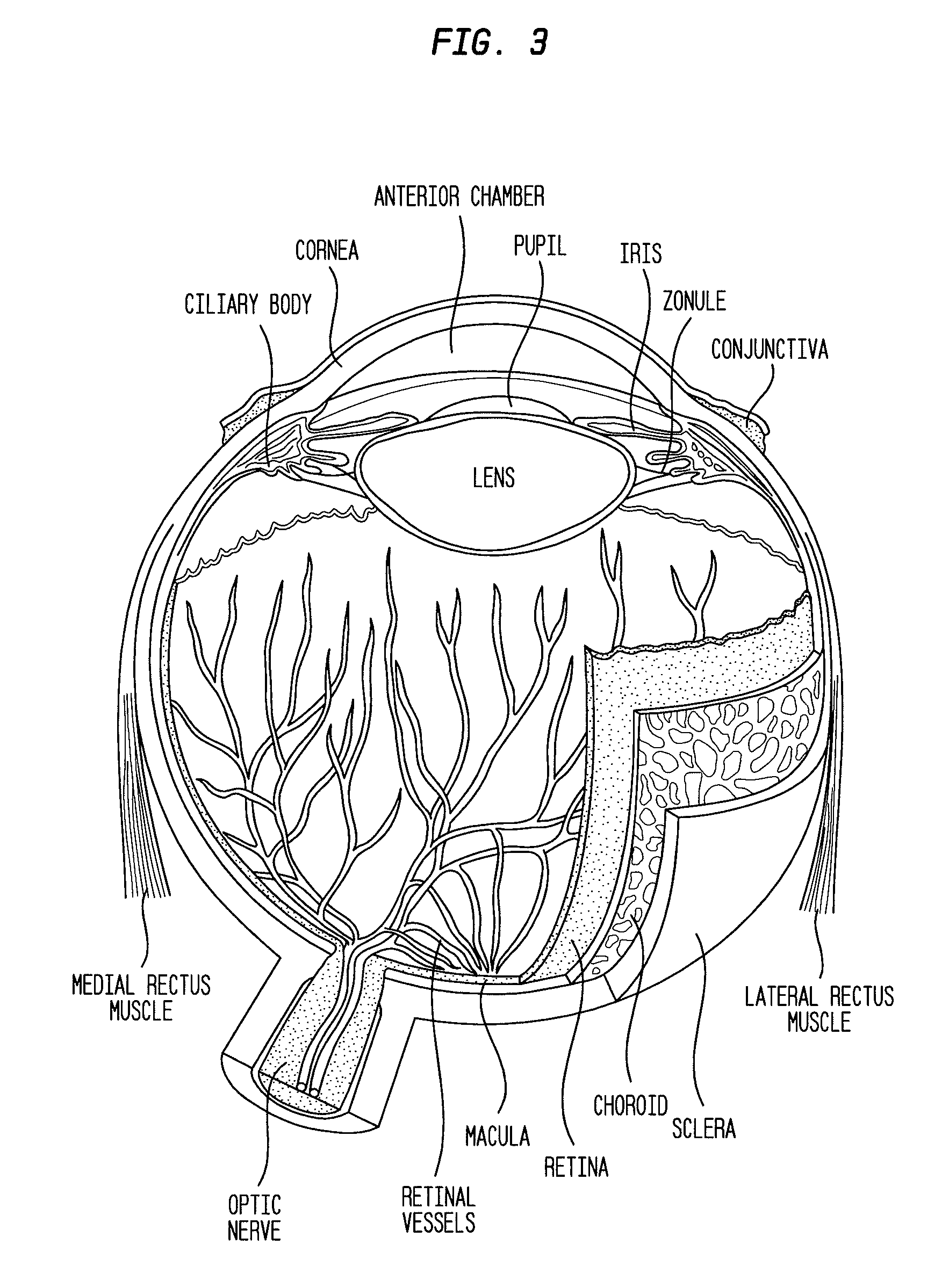
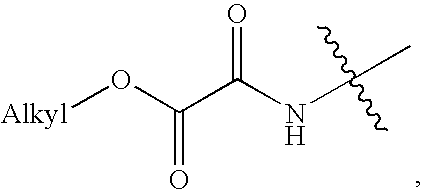
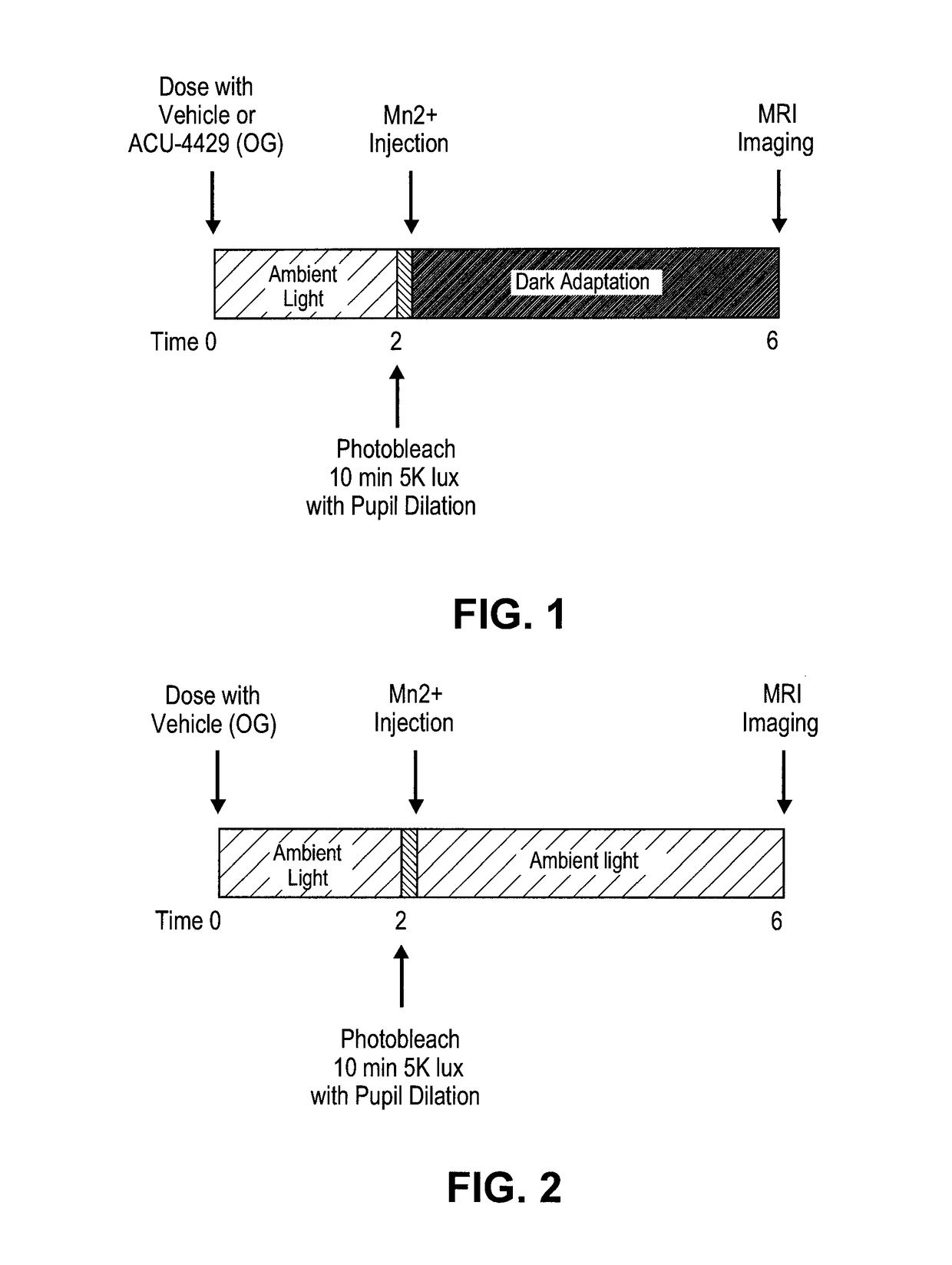
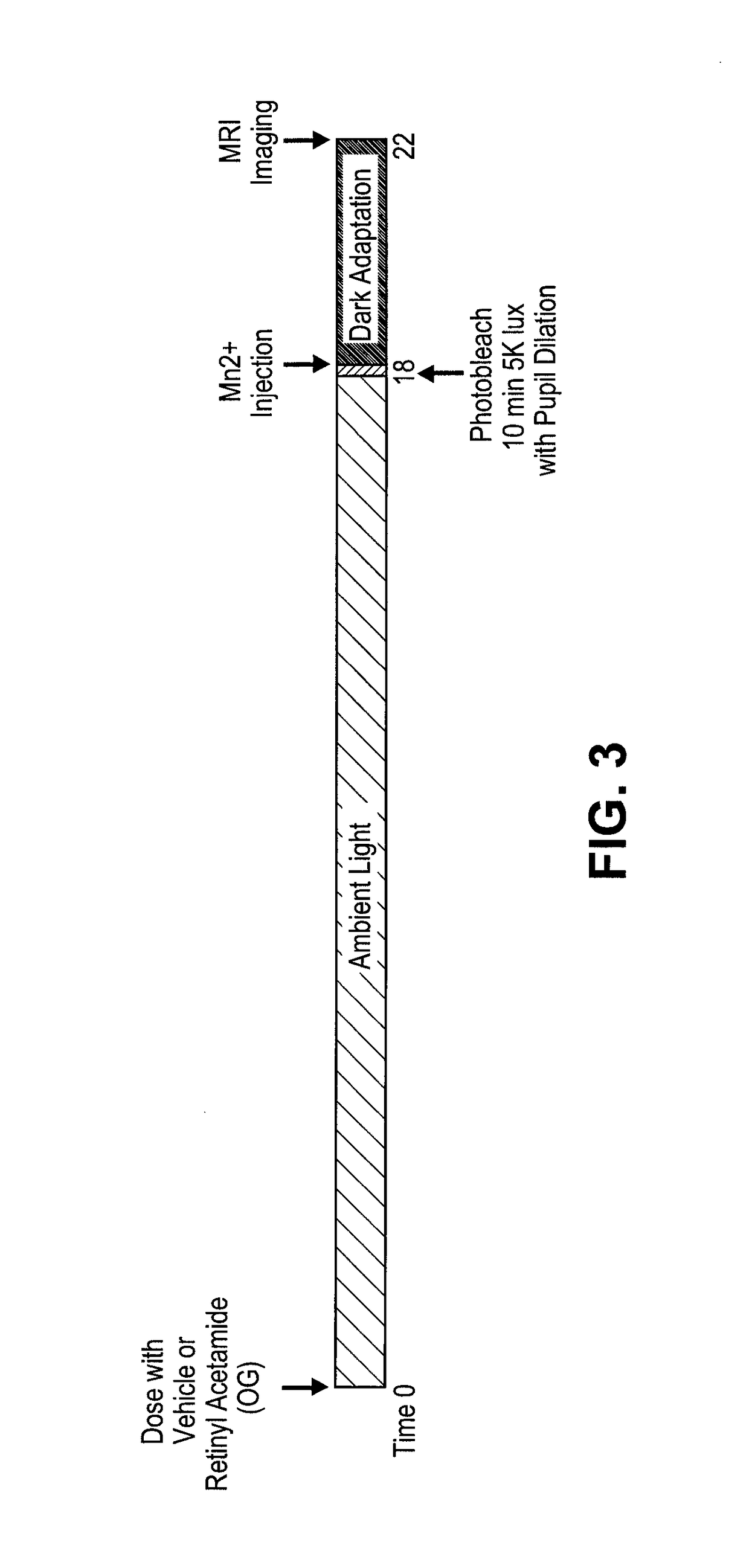
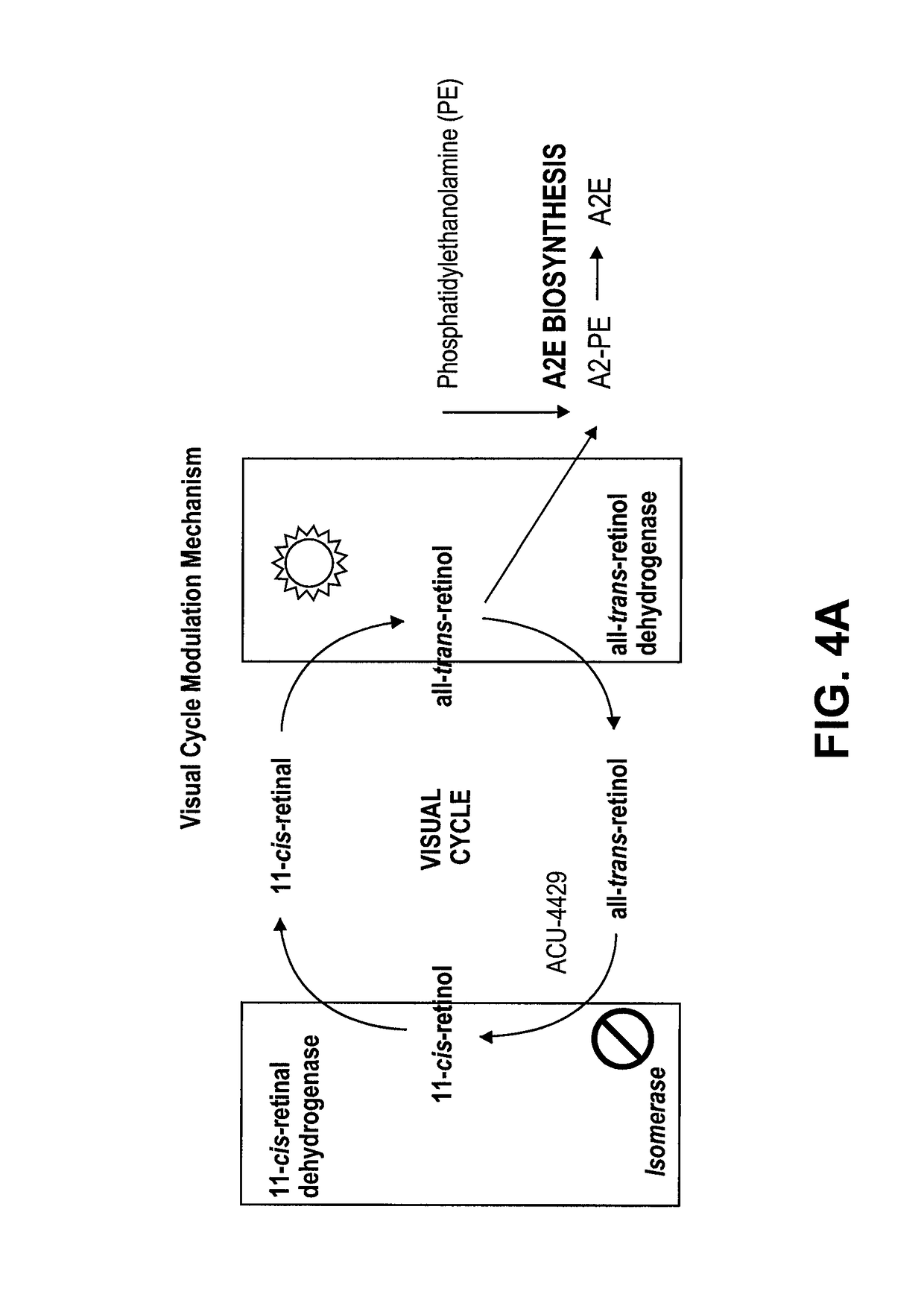
Methods for the treatment of diabetic retinopathy and other ophthalmic diseases
ActiveUS20140039048A1Reduce rod energy demandImprove retinal functionBiocideSenses disorderDiseaseDiabetic retinopathy
Methods are provided herein for the treatment of ophthalmic diseases or conditions such as an ophthalmic disease or disorder associated with diabetes in a patient. Also provided herein are methods of treating retinopathy of prematurity in a patient. Further, provided herein are methods for treating wet age-related macular degeneration in a patient. The methods comprise administration of compounds disclosed herein to a patient in need thereof that inhibit or slow one or more signs or symptoms of such conditions.
Owner:ACUACELA INC
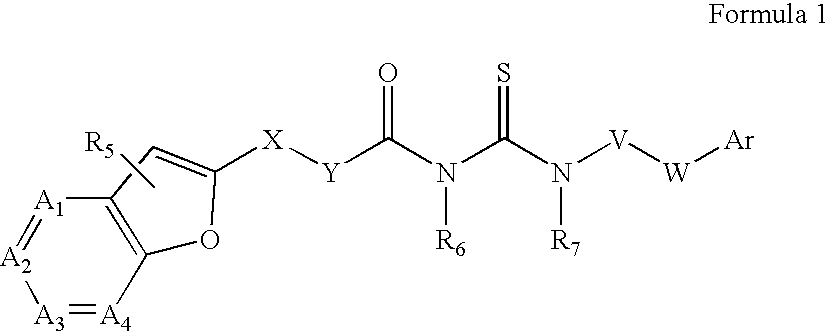
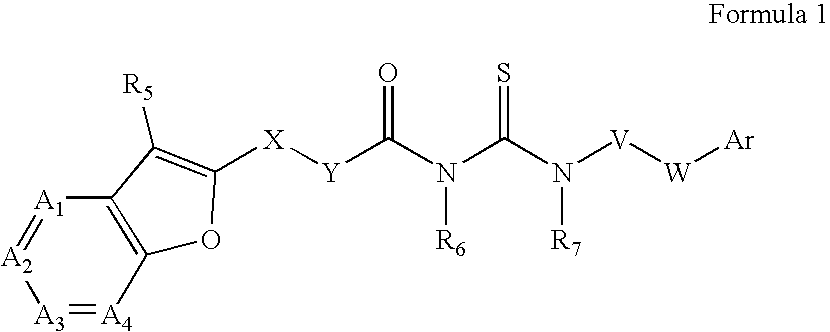
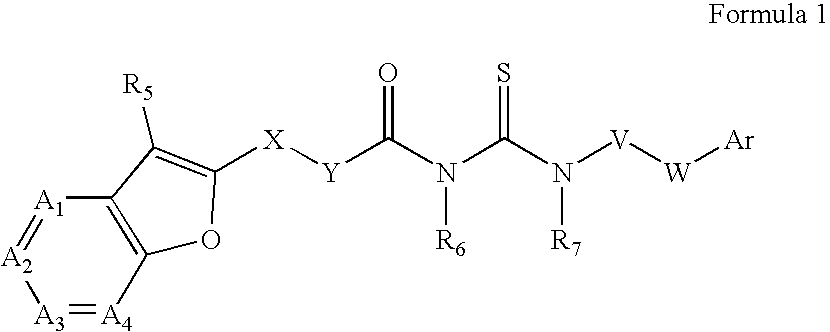
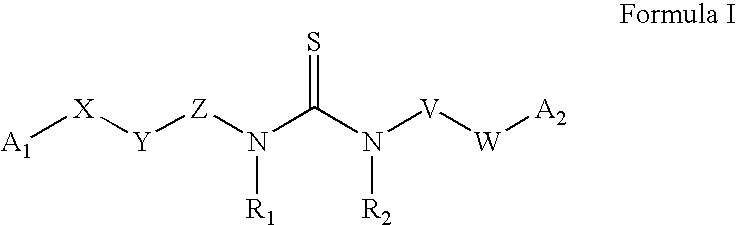
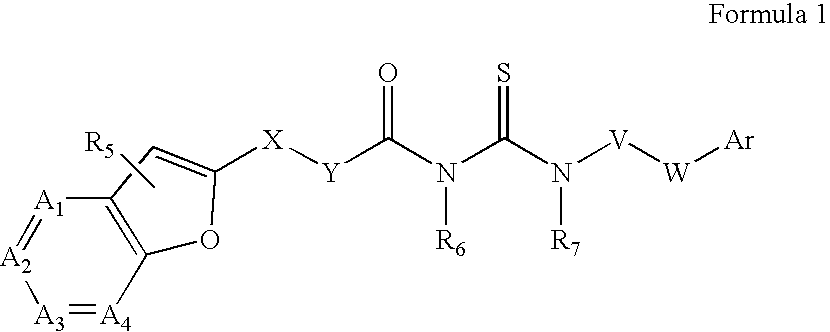
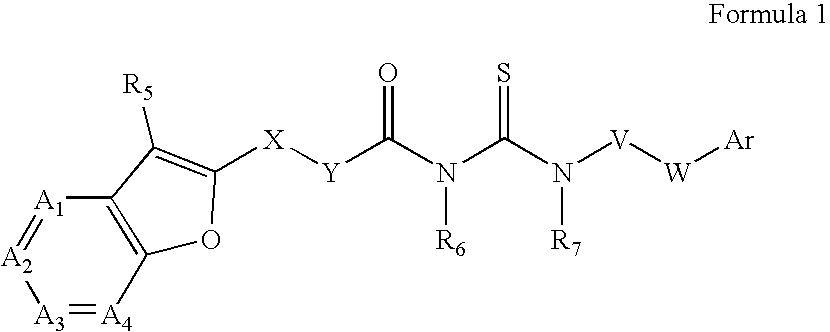
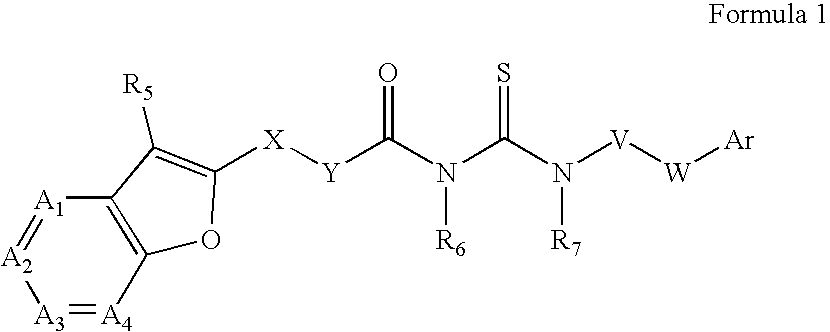
Azabenzofuran substituted thioureas; inhibitors of viral replication
The present invention provides compounds of Formula 1, wherein the variables Ar, A1, A2, A3, A4, R5, R6, R7, V, W. X, and Y are defined herein. Certain compounds of Formula 1 described herein possess potent antiviral activity. The invention also provides compounds of Formula 1 that are potent and / or selective inhibitors of Hepatitis C virus replication. The invention also provides pharmaceutical compositions containing one or more compounds of Formula 1, or a salt, solvate, or acylated prodrug of such compounds, and one or more pharmaceutically acceptable carriers, excipients, or diluents. The invention further comprises methods of treating patients suffering from certain infectious diseases by administering to such patients an amount of a compound of Formula 1 effective to reduce signs or symptoms of the disease. These infectious diseases include viral infections, particularly HCV infections. The invention is particularly includes methods of treating human patients suffering from an infectious disease, but also encompasses methods of treating other animals, including livestock and domesticated companion animals, suffering from an infectious disease. Methods of treatment include administering a compound of Formula 1 as a single active agent or administering a compound of Formula 1 in combination with on or more other therapeutic agent.
Owner:ACHILLION PHARMA INC
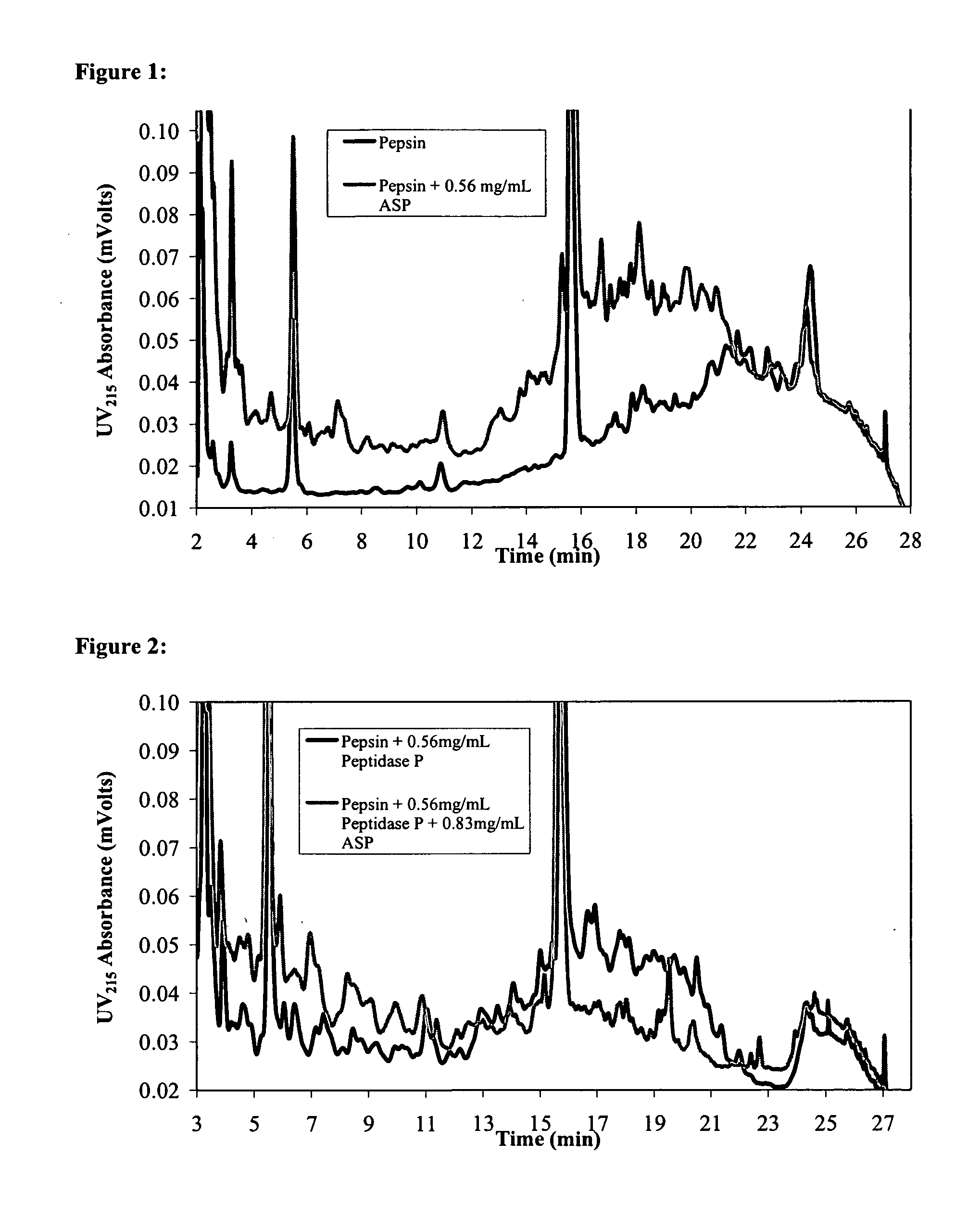
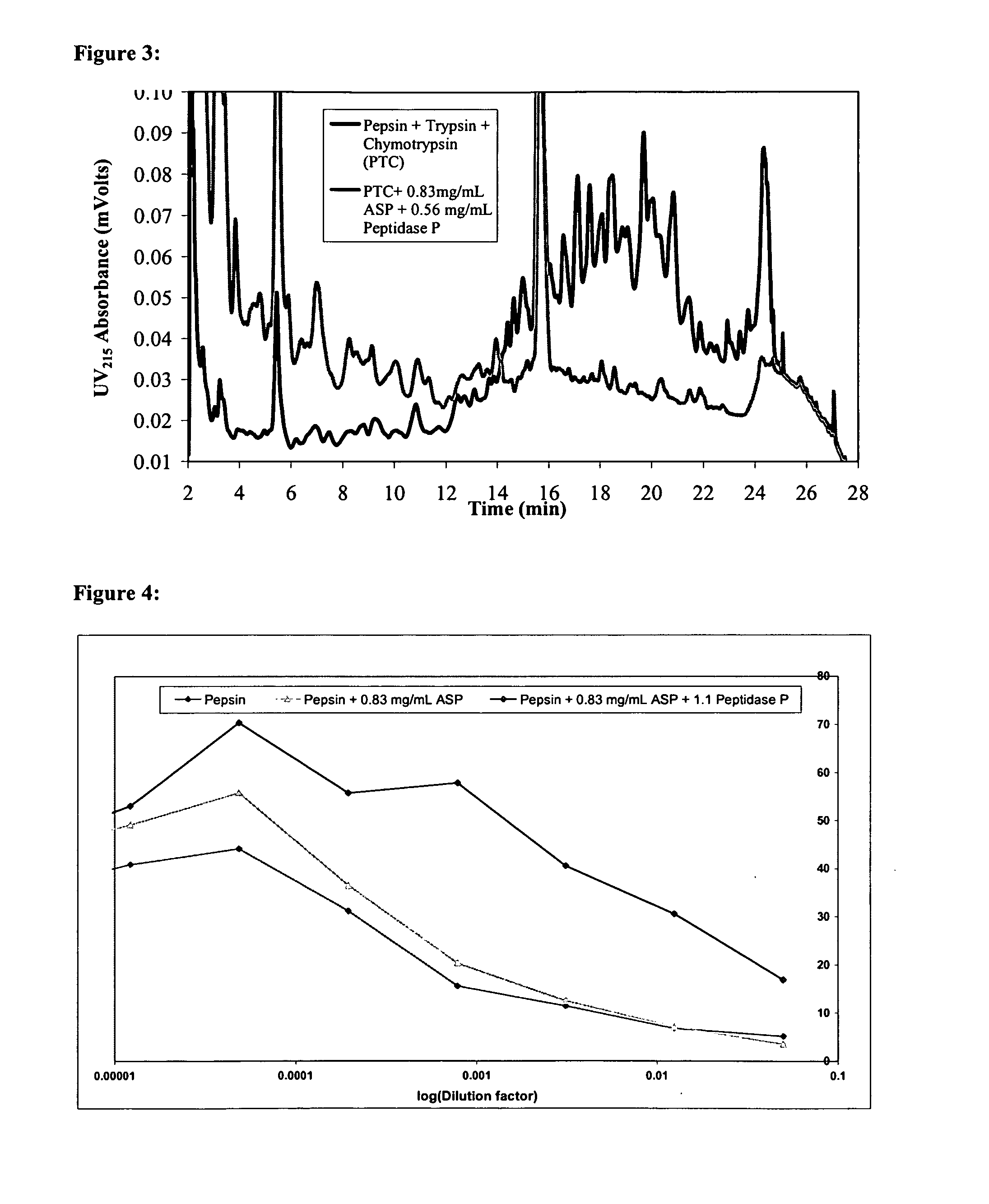
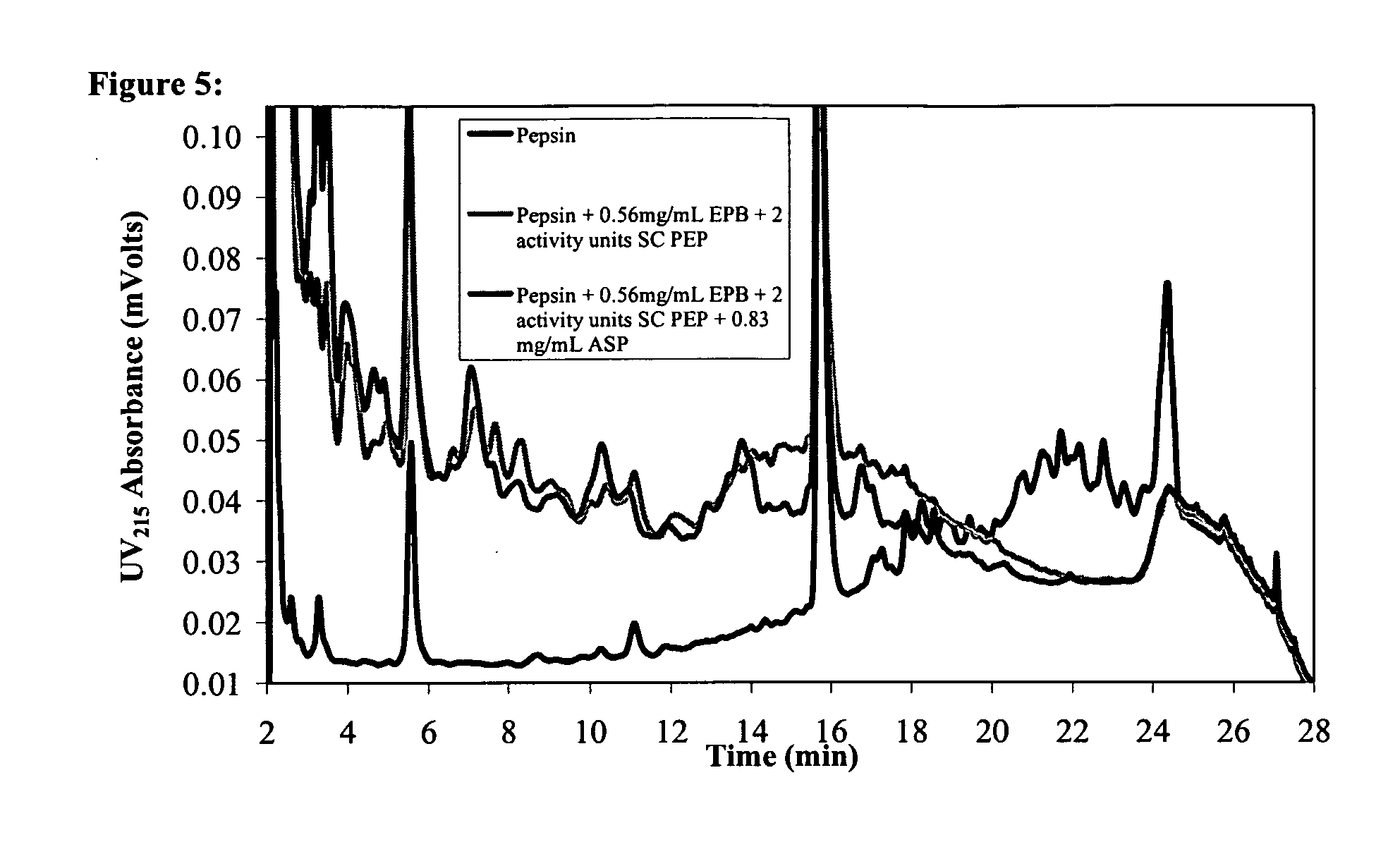
Combination Enzyme Therapy for Gastric Digestion of Dietary Gluten in Celiac Sprue Patients
InactiveUS20100322912A1Decrease in levelGood curative effectPeptide/protein ingredientsDigestive systemGastric digestionProteinase activity
Combination enzyme products and methods of use thereof are provided. Aspergillopepsin I is combined with a protease enzyme that provides for an additive or synergistic effect in the digestion of toxic gluten oligopeptides. The enzyme products are useful in the treatment of Celiac Sprue patients, particularly for patients who continue to exhibit signs or symptoms of active disease despite following a gluten-free diet.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
Curcumin combination with Anti-type 2 diabetic drugs for prevention and treatment of disease sequelae, drug-related adverse reactions, and improved glycemic control
InactiveUS20120237590A1Reduce incidencePrevention loweringBiocidePowder deliveryAdvanced stageDisease injury
Compositions and methods for treating type 2 diabetes and its sequelae by intravenous or subcutaneous administration of formulations of synthesized curcumin (diferuloylmethane) and concomitantly one or more anti-diabetic agents to human subjects are disclosed herein. The composition of the present invention may be used to: (i) treat patients with diabetes in advanced stages with evidence of any or all encephalopathy, retinopathy, nephropathy, pancreatitis or neoplasias; (ii) treat patients with diabetic disease status without symptomatic or pathologic evidence of associated sequelae but requiring better glycemic control than that offered by standard of care anti-diabetic; and (iii) patients with objective signs or symptoms of sequelae from diabetes of anti-diabetic drugs. One three-drug combination of the present invention includes a slow release PLGA-curcumin and an oral gliptin (DPP-4)-inhibitor or any incretin-mimetic and metformin.
Owner:SIGNPATH PHARMA INC
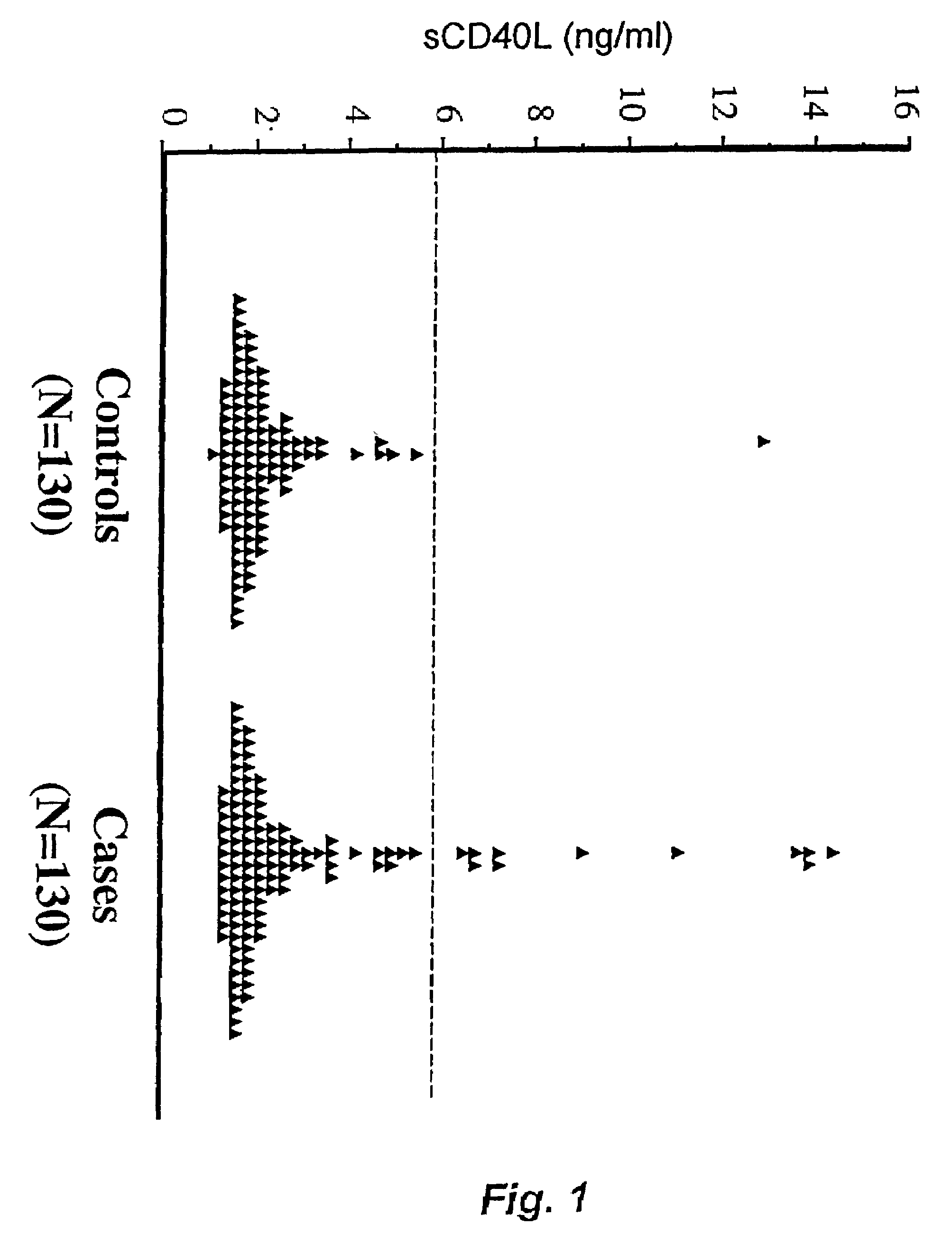
Soluble CD40L(CD154) as a prognostic marker of atherosclerotic diseases
InactiveUS7189518B2Prevent cardiovascular disorderReduce riskBiocidePeptide/protein ingredientsDiagnostic testSoluble cd40l
This invention involves the new use of a diagnostic test to determine the risk of atherosclerotic diseases such as myocardial infarction and stroke, particularly among individuals with no signs or symptoms of current disease and among nonsmokers. Further, this invention involves the new use of a diagnostic test to assist physicians in determining which individuals at risk will preferentially benefit from certain treatments designed either to prevent first or recurrent myocardial infarctions and strokes, or to treat acute and chronic cardiovascular disorders. Methods for treatment also are described.
Owner:THE BRIGHAM & WOMEN S HOSPITAL INC
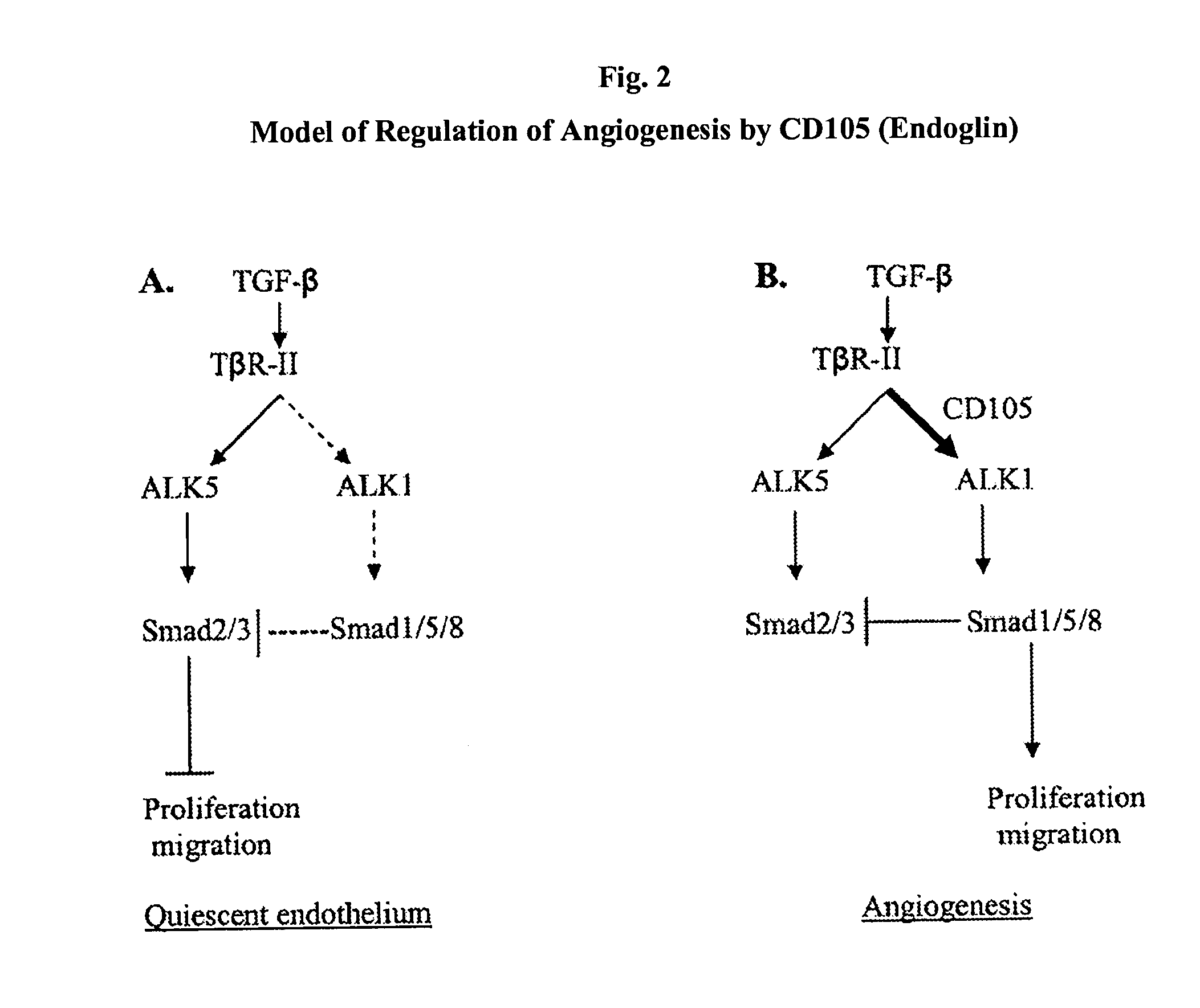
Antibody Formulations and Uses Thereof
ActiveUS20150209430A1Prevent proliferationImprove eyesightSenses disorderPharmaceutical delivery mechanismDiseaseAntigen Binding Fragment
The present application relates to formulations of anti-CD105 antibodies, antigen-binding fragments thereof, and uses thereof. Another aspect relates to pre-filled syringes of the formulations of anti-CD105 antibodies or antigen-binding fragments thereof. Another aspect relates to the use of the formulations to reduce one or more signs or symptoms of an angiogenesis-related disorder such as cancers and ophthalmologic diseases.
Owner:KAIROS PHARMA LTD
Method of treating bone metastasis
Owner:TRUSTEES OF DARTMOUTH COLLEGE THE
Compositions and methods for reducing visual loss
InactiveUS20160346224A1Reduce visual lossOrganic active ingredientsSenses disorderParticulatesSide effect
The described invention provides a method for reducing visual loss and for treating one or more of adverse consequence of an eye disease, including abnormal intraocular pressure, retinal vascular disease, retinal ganglion cell death, or a combination thereof in order to reduce visual loss. The method entails providing a flowable particulate composition that contains a particulate formulation comprising a plurality of particles of uniform size distribution, a therapeutic amount of a therapeutic agent selected from a voltage-gated calcium channel antagonist, an endothelin receptor antagonist, or a combination thereof, and optionally an additional therapeutic agent, wherein the particles are of uniform size distribution, and wherein each particle comprises a matrix; and a pharmaceutically acceptable carrier. The pharmaceutical composition is characterized by: dispersal of the therapeutic agent throughout each particle, adsorption of the therapeutic agent onto the particles, or placement of the therapeutic agent in a core surrounded by a coating, sustained release of the therapeutic agent and optionally the additional therapeutic agent from the composition, and a local therapeutic effect that is effective to reduce signs or symptoms of the adverse consequence without entering systemic circulation in an amount to cause unwanted side effects. The method further entails administering a therapeutic amount of the pharmaceutical composition by a means for administration at a site of administration. The administering includes topically, parenterally, or by implantation. Sites of administration include intraocularly, intraorbitally, or into subconjunctival space.
Owner:EDGE THERAPEUTICS
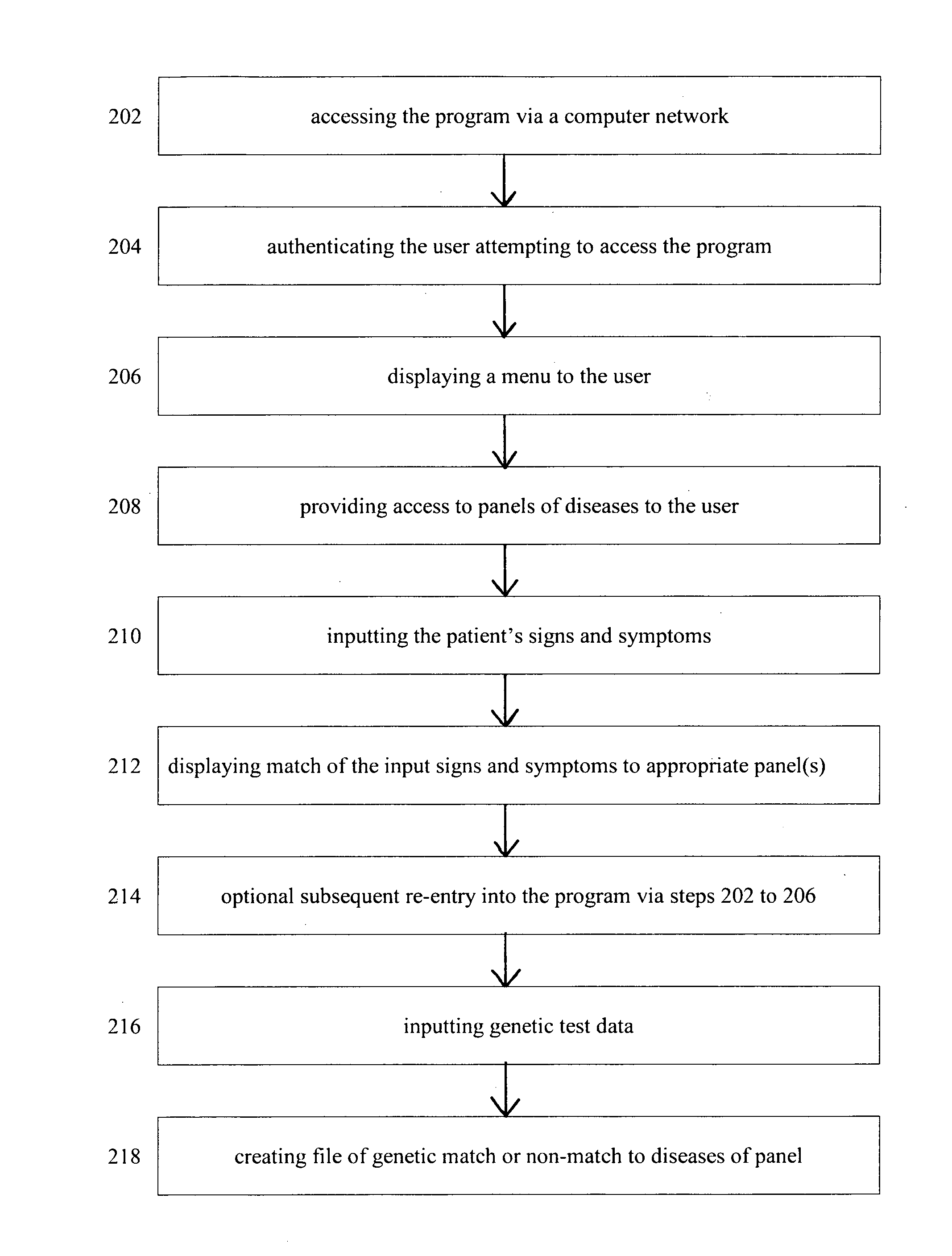
Multiple genetic disease diagnostic panels by one single test using microarray technology
InactiveUS20120021943A1Increase costShorten the timeNucleotide librariesMicrobiological testing/measurementBiologySign or Symptom
The invention utilizes clinical features of diseases with a genetic background to define logical panels of diseases which have shared signs or symptoms. The invention includes methods for collecting data for use in determining a cause or risk factor for disease and includes micro arrays for use in detecting mutations associated with the diseases set forth in the panel.
Owner:CGC CENT DE GENETICA CLINICA
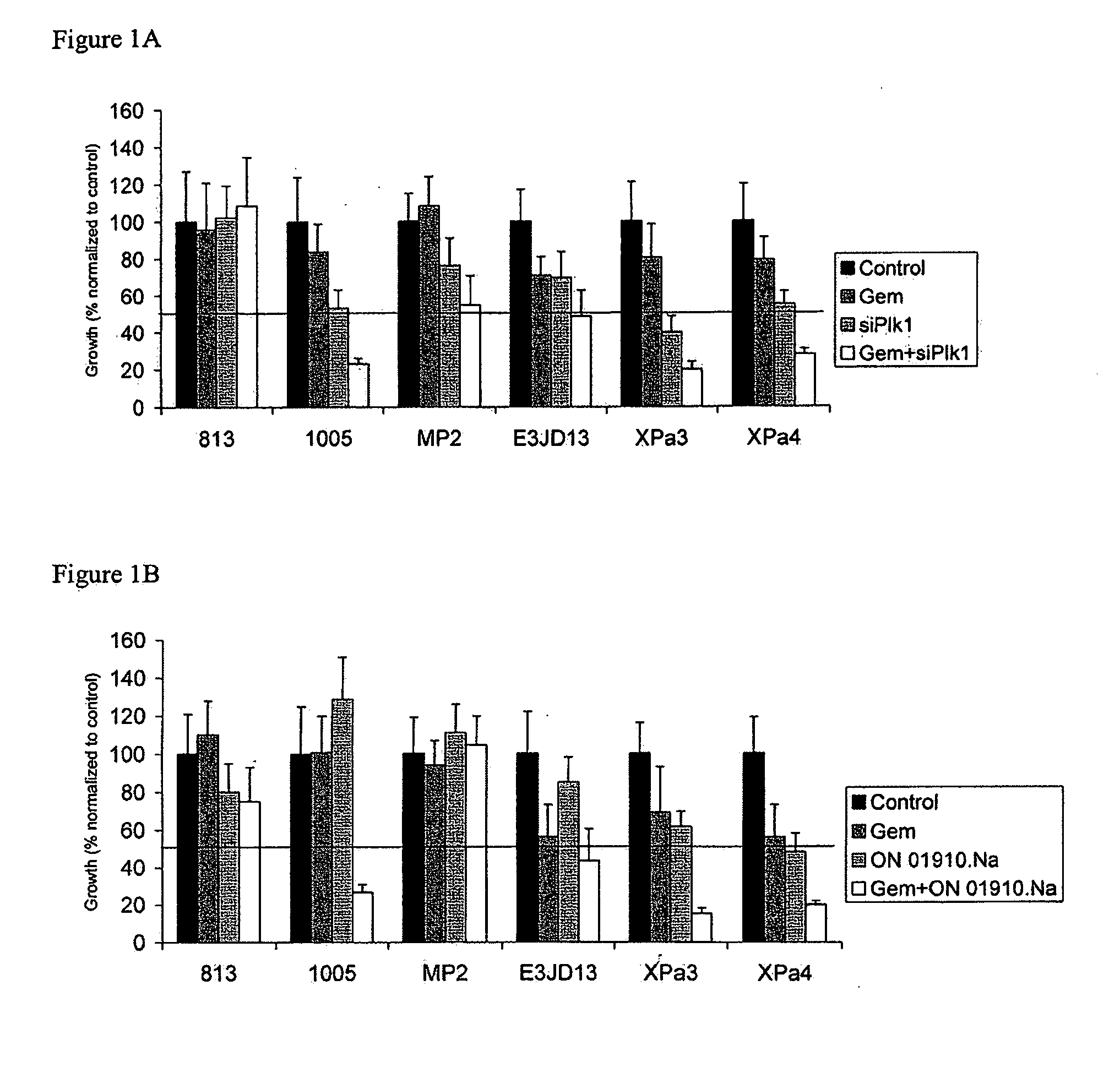
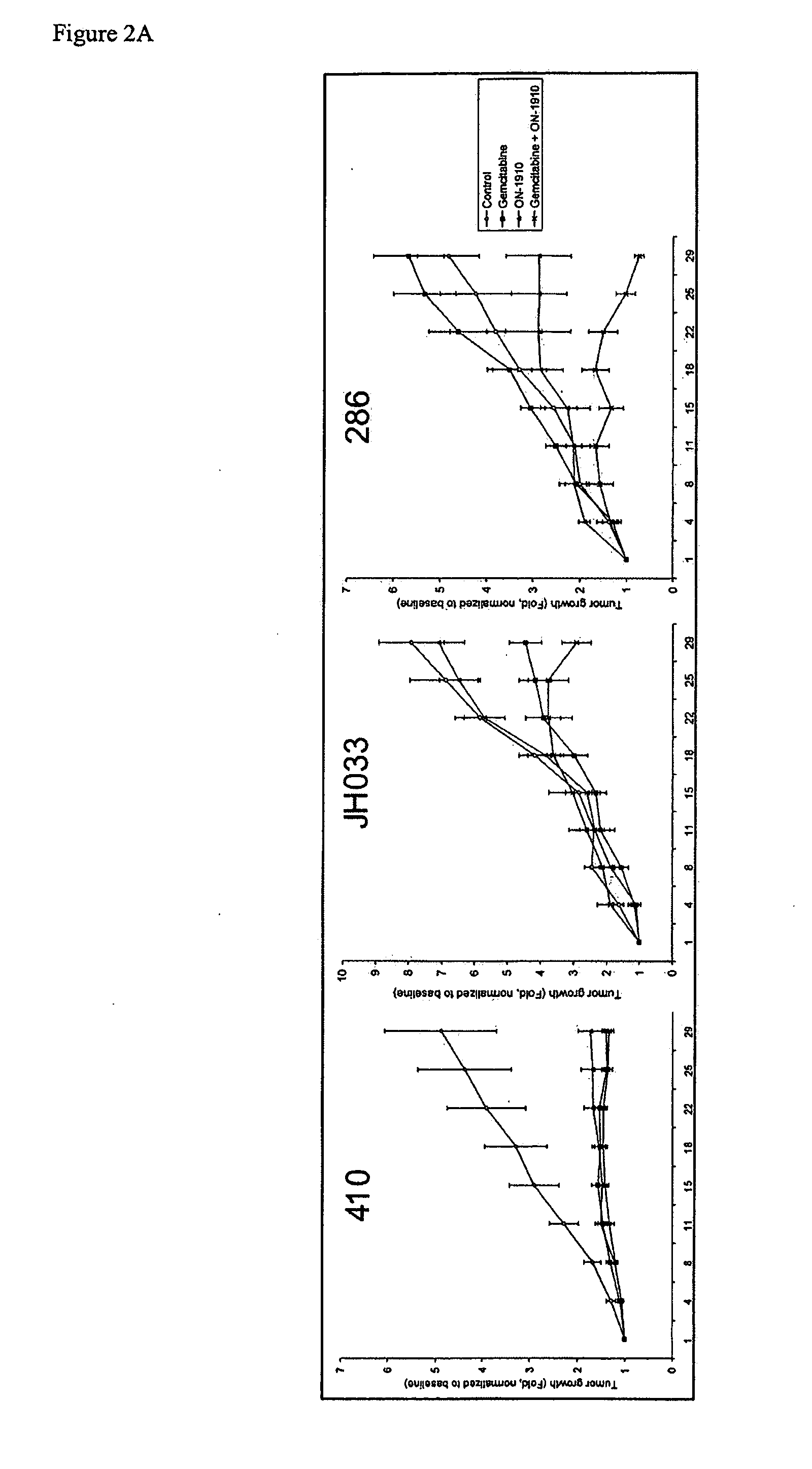
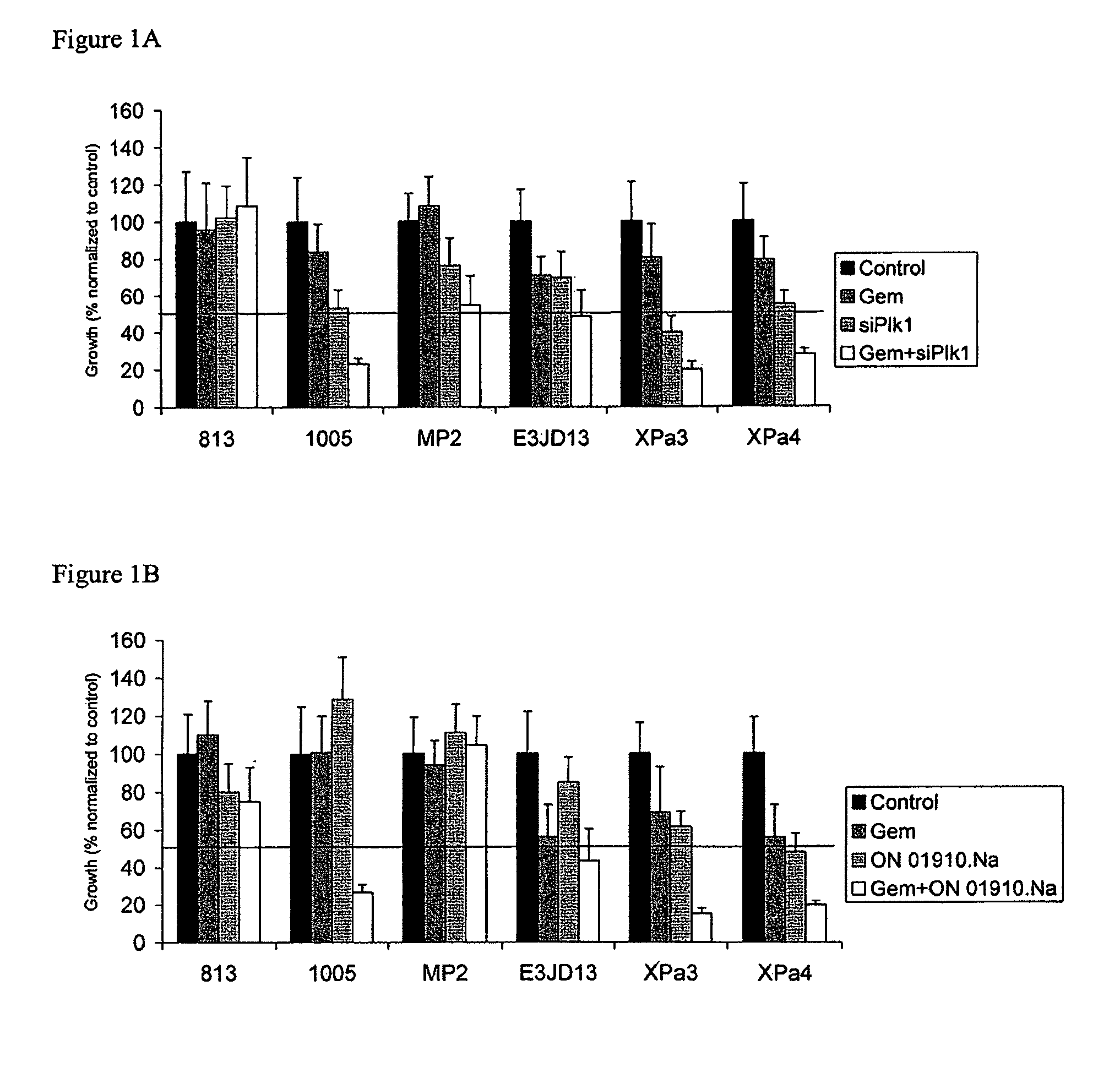
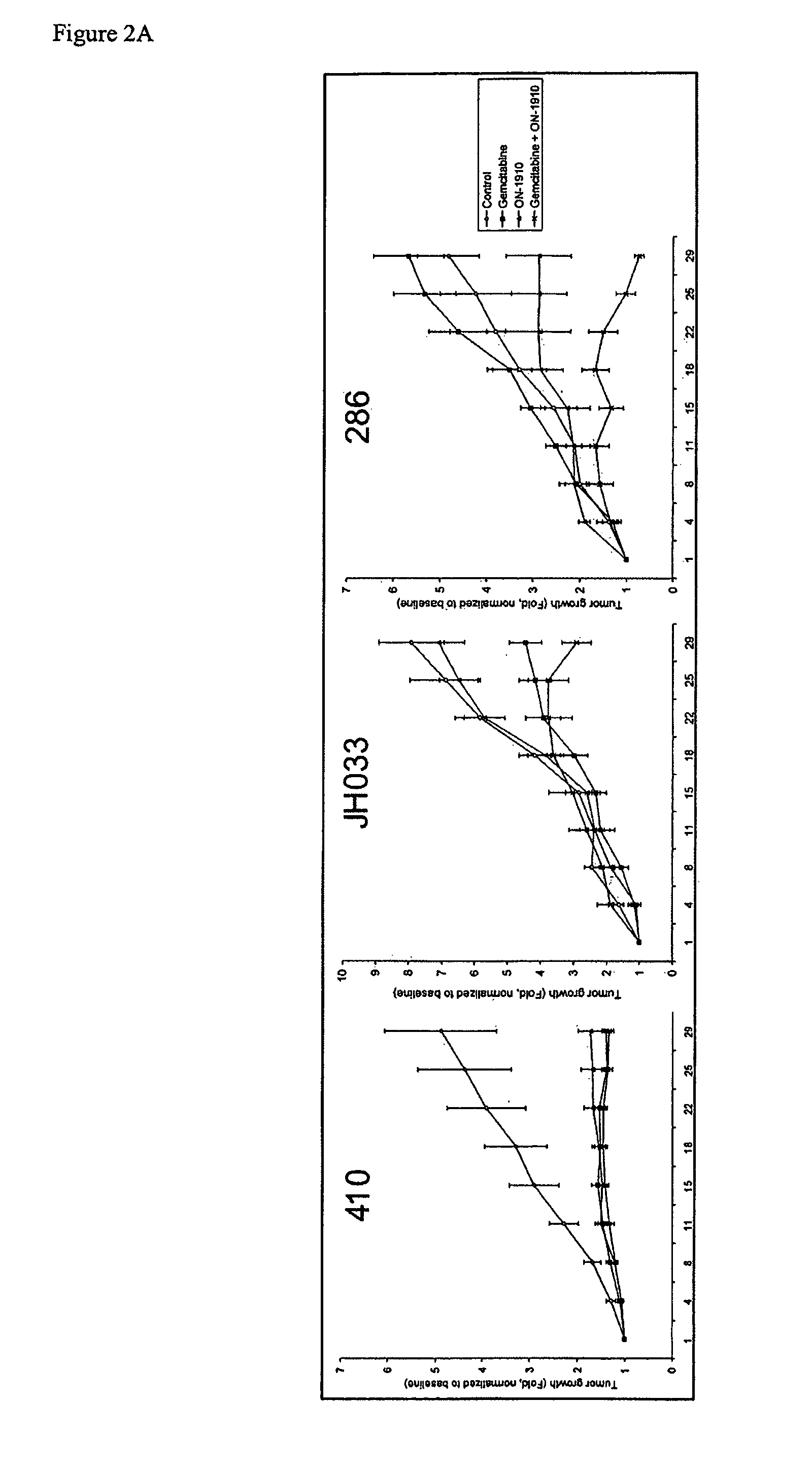
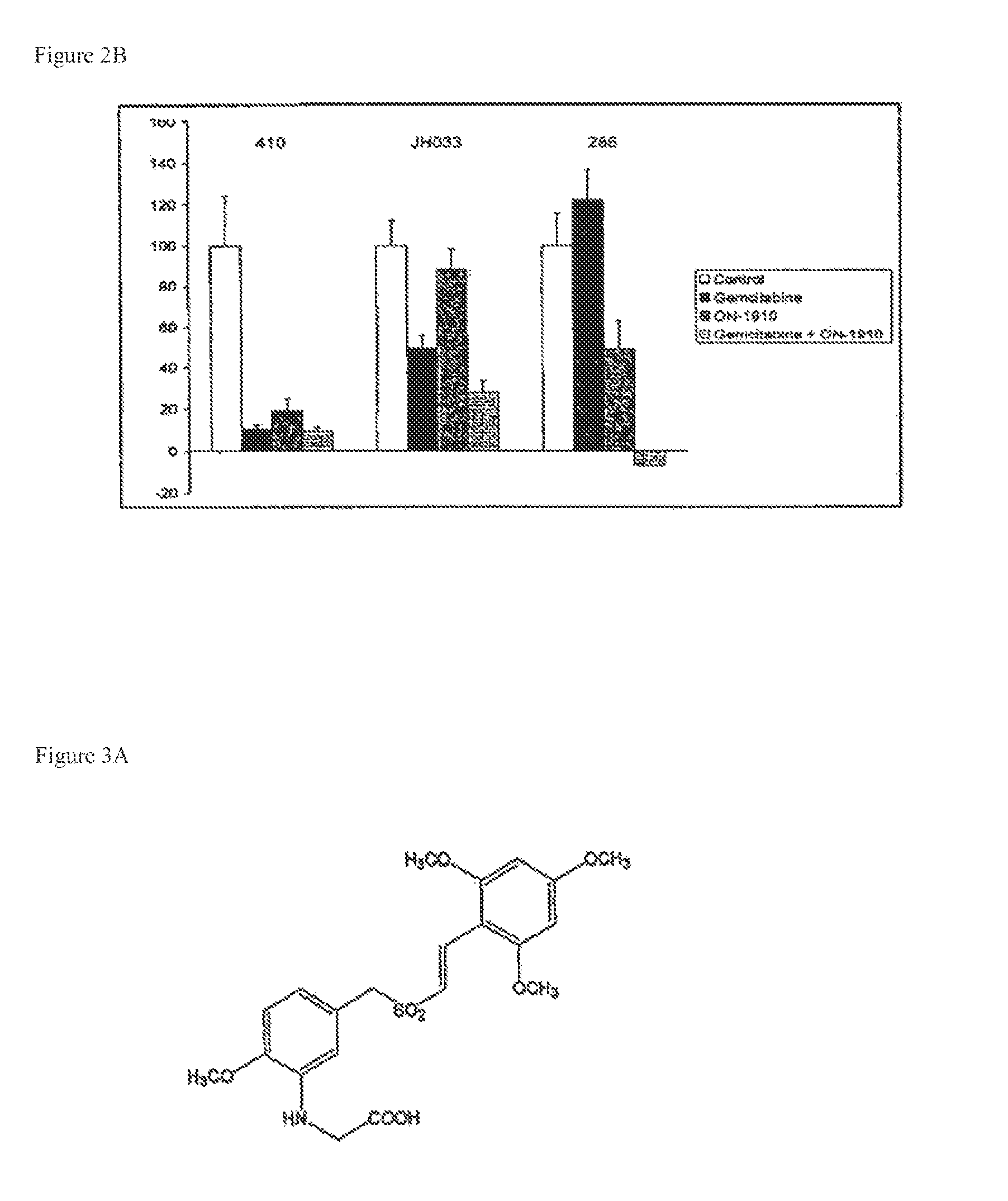
ON01910.Na ENHANCES CHEMOTHERAPEUTIC AGENT ACTIVITY IN DRUG-RESISTANT TUMORS
InactiveUS20110201675A1Good effectAvoid developmentBiocidePeptide/protein ingredientsNucleotidePolo-like kinase
The invention includes compositions and methods of treatment of cancers susceptible to treatment with nucleotide analog chemotherapeutic agent, including cancers in which nucleotide analog resistant tumors have developed, including identifying a subject having cancer susceptible to treatment with a nucleotide analog chemotherapeutic agent and a mitotic disruptor / polo-like kinase (Plk) pathway inhibitor to a subject; and monitoring the subject for a reduction or stabilization of at least one sign or symptom of cancer.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
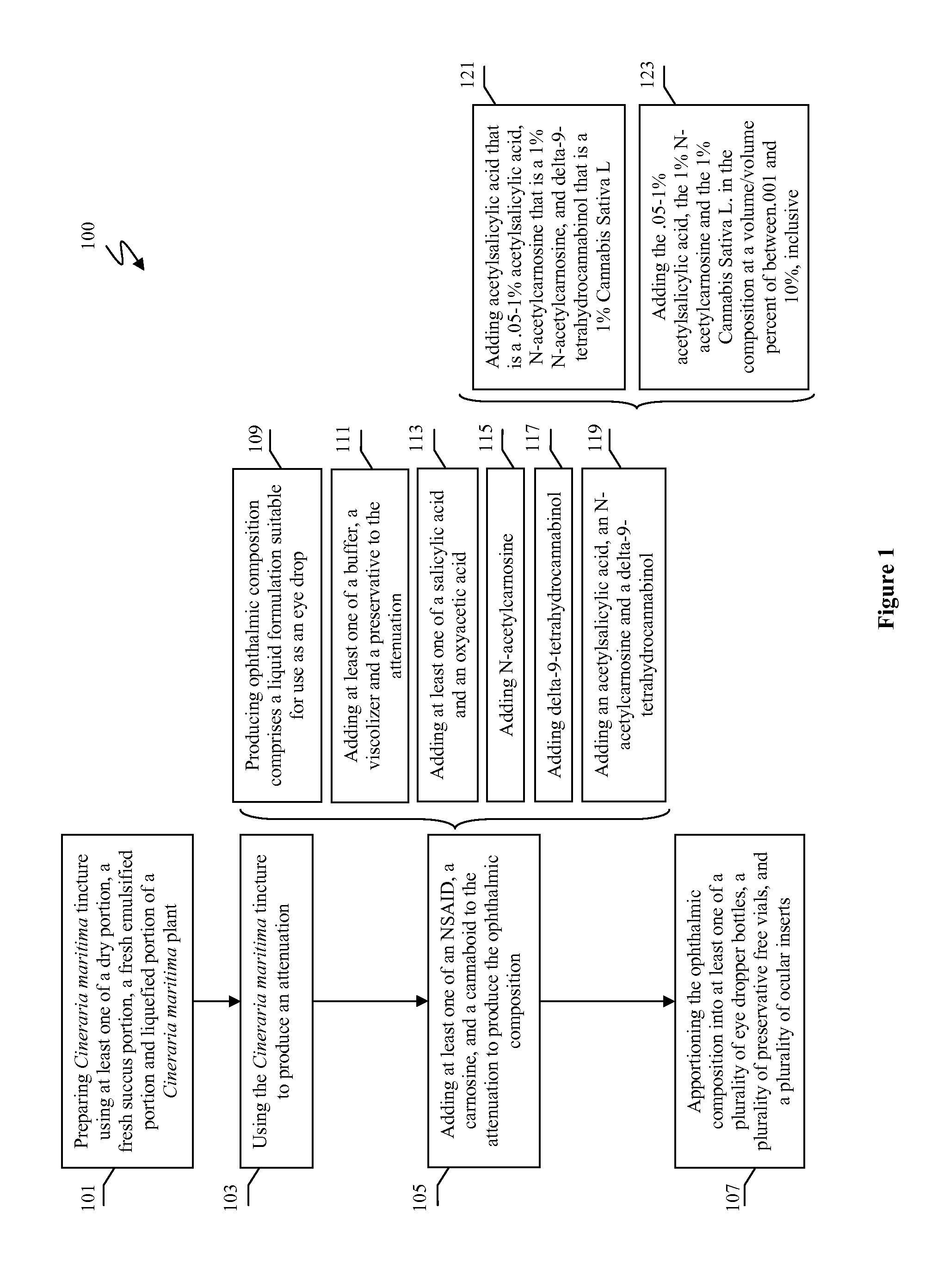
Methods and compositions for treating and preventing signs or symptoms of eye disease
ActiveUS20150099019A1BiocideOrganic active ingredientsCannabinoidNonsteroidal Antiinflammatory Drugs/NSAIDs
Compositions and methods for the treatment and prevention of at least one of a sign and a symptom associated with an eye disease are disclosed. Preferred compositions could comprise a liquid formulation including Cineraria maritima and at least one of a nonsteroidal anti-inflammatory drug, a carnosine, and a cannabinoid.
Owner:JOHNSON LIVING TRUST DATED OCTOBER 26 2001 LEONIDAS A JOHNSON TRUSTEE
Substituted aryl acylthioureas and related compounds; inhibitors of viral replication
The invention provides compounds and pharmaceutically acceptable salts of Formula Iwherein the variables A1, A2, R1, R2, V, W, X, Y, and Z are defined herein. Certain compounds of Formula I described herein which possess potent antiviral activity. The invention particularly provides compounds of Formula I that are potent and / or selective inhibitors of Hepatitis C virus replication. The invention also provides pharmaceutical compositions containing one or more compound of Formula I, or a salt, solvate, or acylated prodrug of such compounds, and one or more pharmaceutically acceptable carriers, excipients, or diluents.The invention further comprises methods of treating patients suffering from certain infectious diseases by administering to such patients an amount of a compound of Formula I effective to reduce signs or symptoms of the disease or disorder. These infectious diseases include viral infections, particularly HCV infections. The invention is particularly includes methods of treating human patients suffering from an infectious disease, but also encompasses methods of treating other animals, including livestock and domesticated companion animals, suffering from an infectious disease.Methods of treatment include administering a compound of Formula I as a single active agent or administering a compound of Formula I in combination with on or more other therapeutic agent.
Owner:ACHILLION PHARMA INC
Methods for the treatment of diabetic retinopathy and other ophthalmic diseases
ActiveUS9957224B2Reduce rod energy demandImprove retinal functionSenses disorderOrganic chemistryDiseaseDiabetic retinopathy
Owner:ACUACELA INC
Heteroaryl guanidines; inhibitors of viral replication
Compounds of Formula 1, and pharmaceutically acceptable forms thereof, are provided: wherein the variables X, Y, Z, A1, R2, R3, R4, R5 and R6 are defined herein. Certain compounds of Formula 1 described herein which possess potent antiviral activity. Certain compounds of Formula 1 that are potent and / or selective inhibitors of Hepatitis C virus replication. Pharmaceutical compositions containing one or more compounds of Formula 1, or a salt, solvate, or acylated prodrug of such compounds, and one or more pharmaceutically acceptable carriers, excipients, or diluents are also provided. Methods of treating patients suffering from certain infectious diseases by administering to such patients an amount of a compound of Formula 1 effective to reduce signs or symptoms of the disease or disorder are disclosed. These infectious diseases include viral infections, particularly HCV infections. Methods of treating human patients suffering from an infectious disease, but also encompasses methods of treating other animals, including livestock and domesticated companion animals, suffering from an infectious disease. Methods of treatment include administering a compound of Formula 1 as a single active agent or administering a compound of Formula 1 in combination with on or more other therapeutic agent.
Owner:ACHILLION PHARMA INC
Azabenzofuran substituted thioureas; inhibitors of viral replication
The present invention provides compounds of Formula 1,wherein the variables Ar, A1, A2, A3, A4, R5, R6, R7, V, W, X, and Y are defined herein.Certain compounds of Formula 1 described herein possess potent antiviral activity. The invention also provides compounds of Formula 1 that are potent and / or selective inhibitors of Hepatitis C virus replication. The invention also provides pharmaceutical compositions containing one or more compounds of Formula 1, or a salt, solvate, or acylated prodrug of such compounds, and one or more pharmaceutically acceptable carriers, excipients, or diluents. The invention further comprises methods of treating patients suffering from certain infectious diseases by administering to such patients an amount of a compound of Formula 1 effective to reduce signs or symptoms of the disease. These infectious diseases include viral infections, particularly HCV infections. The invention is particularly includes methods of treating human patients suffering from an infectious disease, but also encompasses methods of treating other animals, including livestock and domesticated companion animals, suffering from an infectious disease.Methods of treatment include administering a compound of Formula 1 as a single active agent or administering a compound of Formula 1 in combination with on or more other therapeutic agent.
Owner:ACHILLION PHARMA INC
Substituted aryl thioureas and related compounds; inhibitors of viral replication
The invention provides compounds and pharmaceutically acceptable salts of Formula I wherein the variables A1, A2, R1, R2, V, W, X, Y, and Z are defined herein. Certain compounds of Formula I described herein which possess potent antiviral activity. The invention particularly provides compounds of Formula I that are potent and / or selective inhibitors of Hepatitis C virus replication. The invention also provides pharmaceutical compositions containing one or more compound of Formula I, or a salt, solvate, or acylated prodrug of such compounds, and one or more pharmaceutically acceptable carriers, excipients, or diluents. The invention further comprises methods of treating patients suffering from certain infectious diseases by administering to such patients an amount of a compound of Formula I effective to reduce signs or symptoms of the disease or disorder. These infectious diseases include viral infections, particularly HCV infections. The invention is particularly includes methods of treating human patients suffering from an infectious disease, but also encompasses methods of treating other animals, including livestock and domesticated companion animals, suffering from an infectious disease. Methods of treatment include administering a compound of Formula I as a single active agent or administering a compound of Formula I in combination with on or more other therapeutic agent.
Owner:ACHILLION PHARMA INC
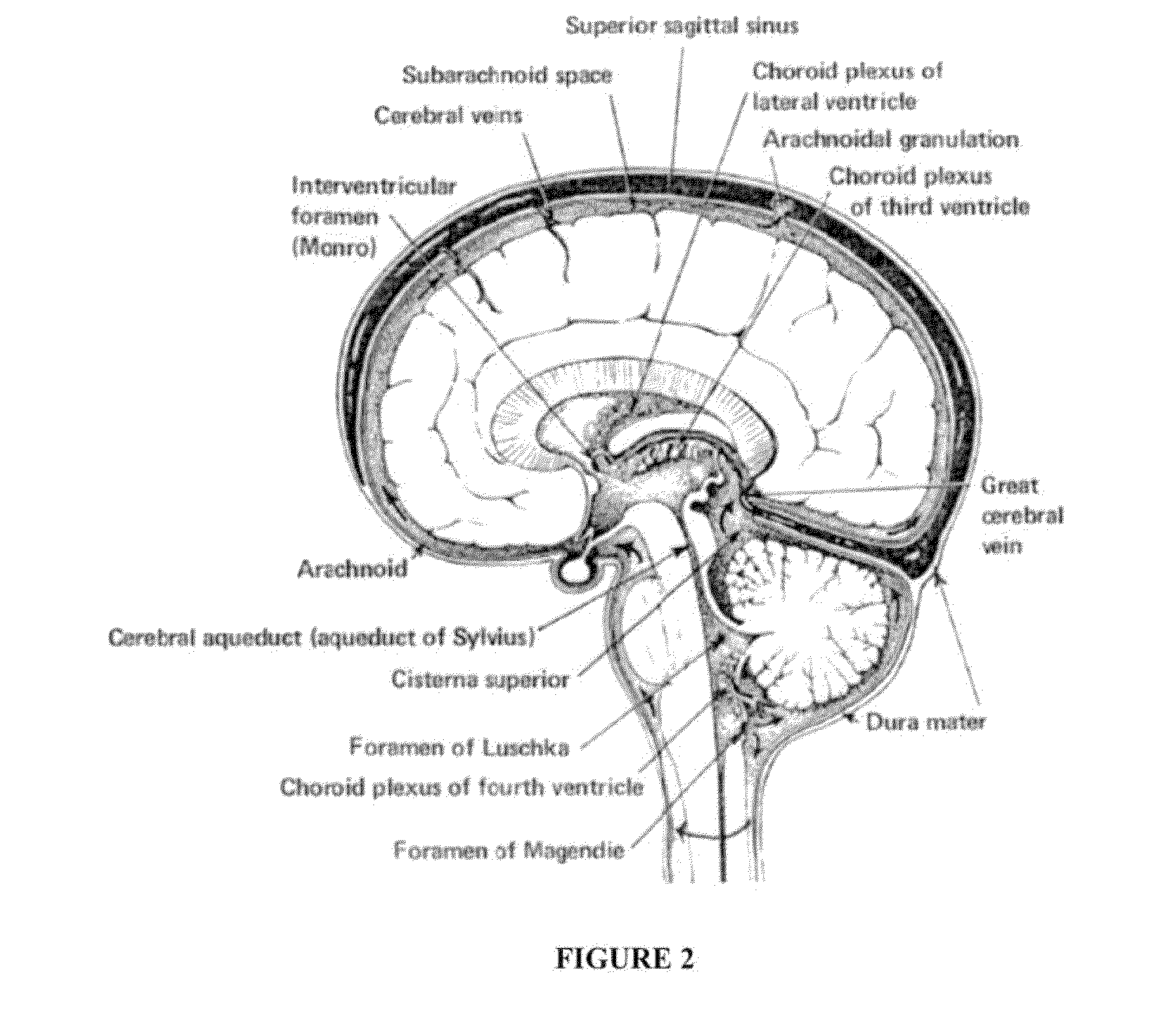
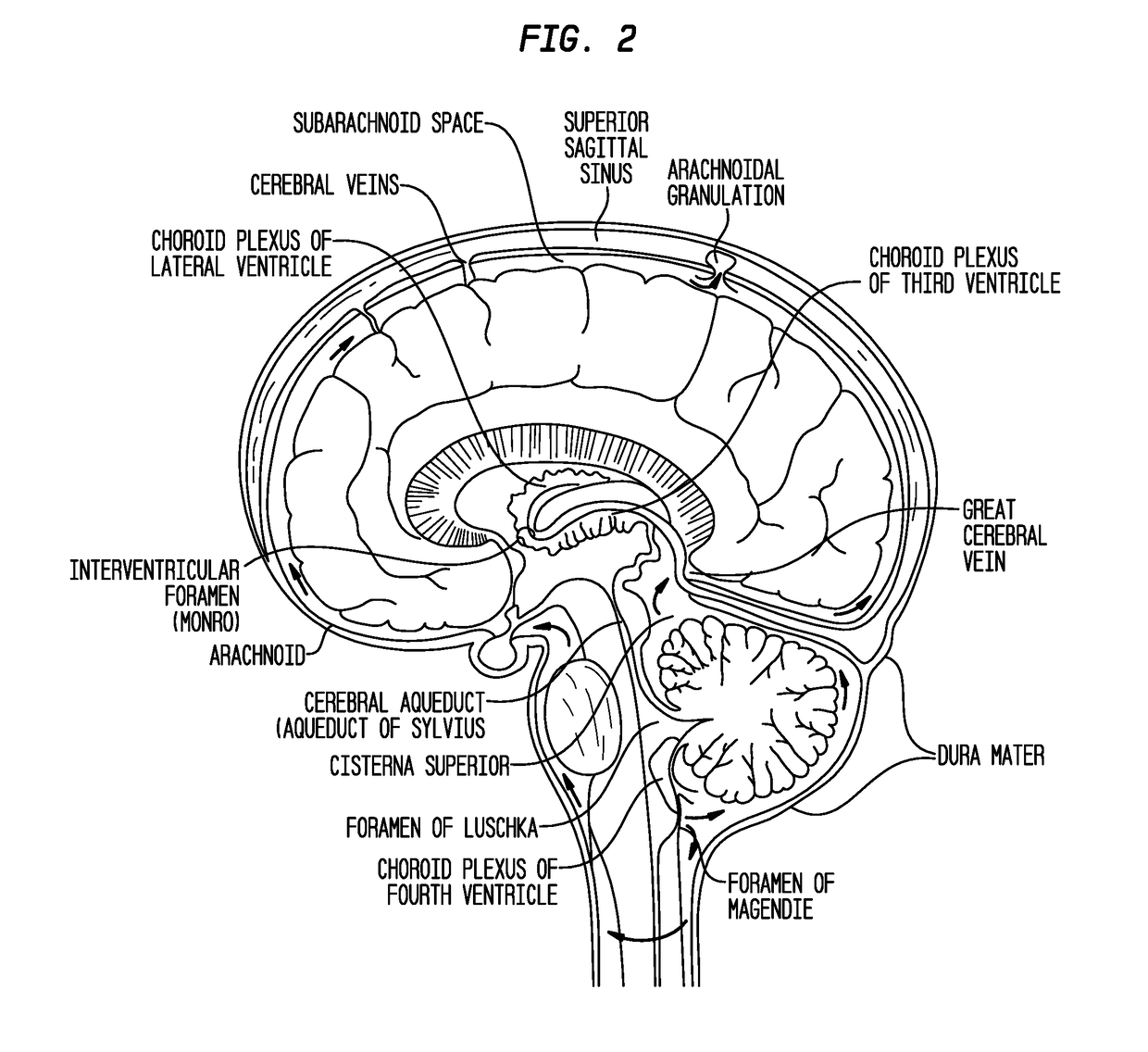
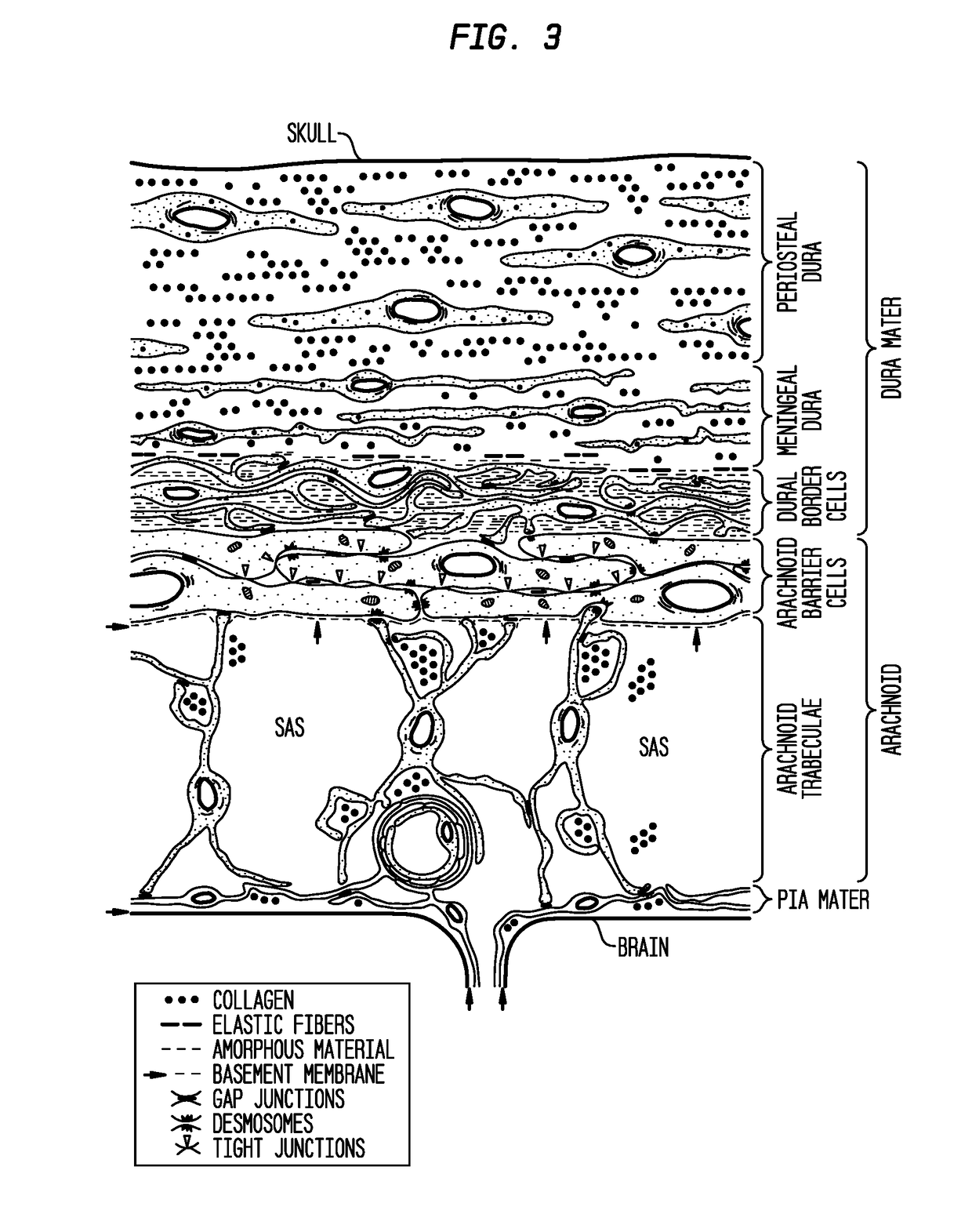
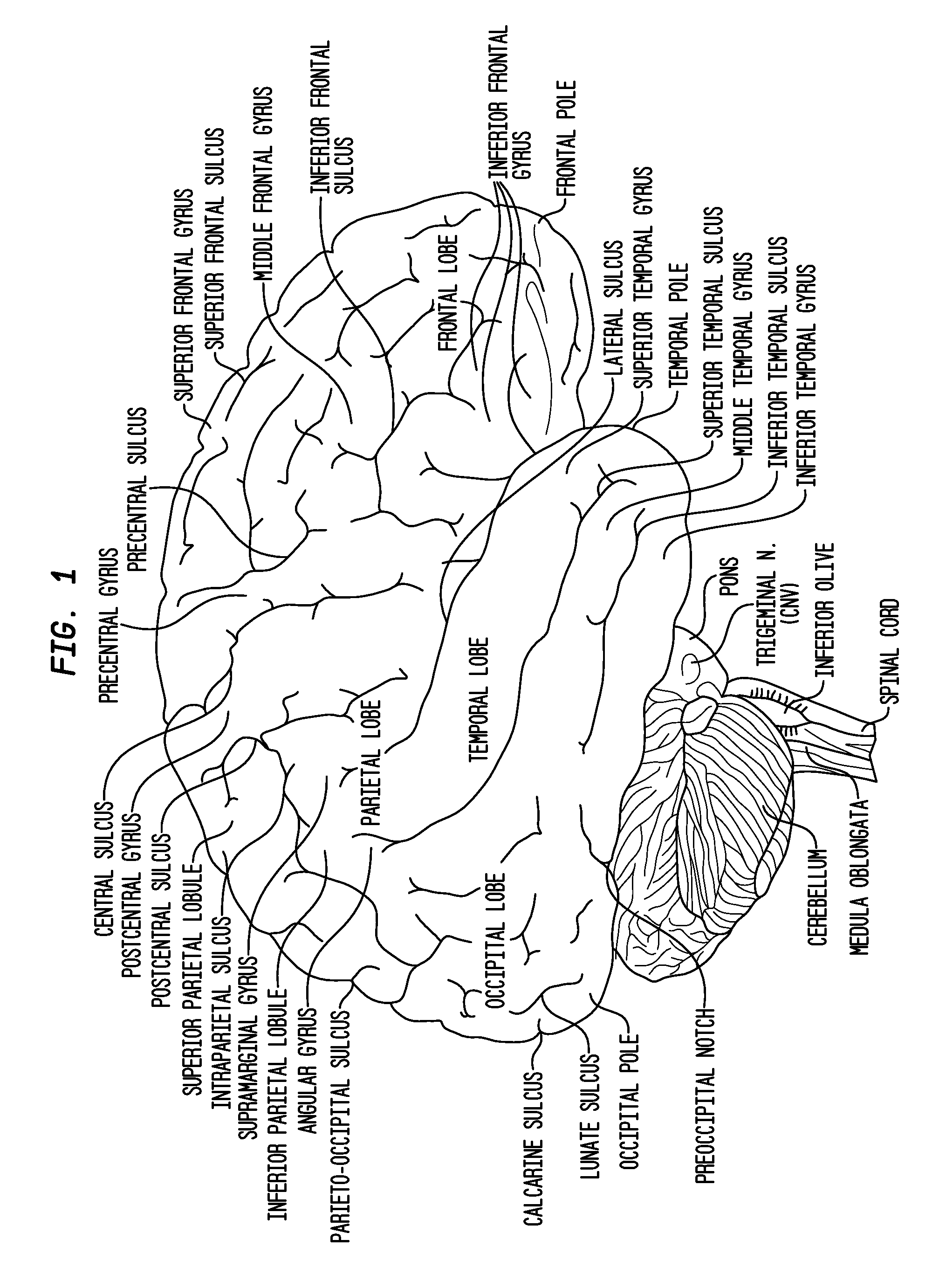
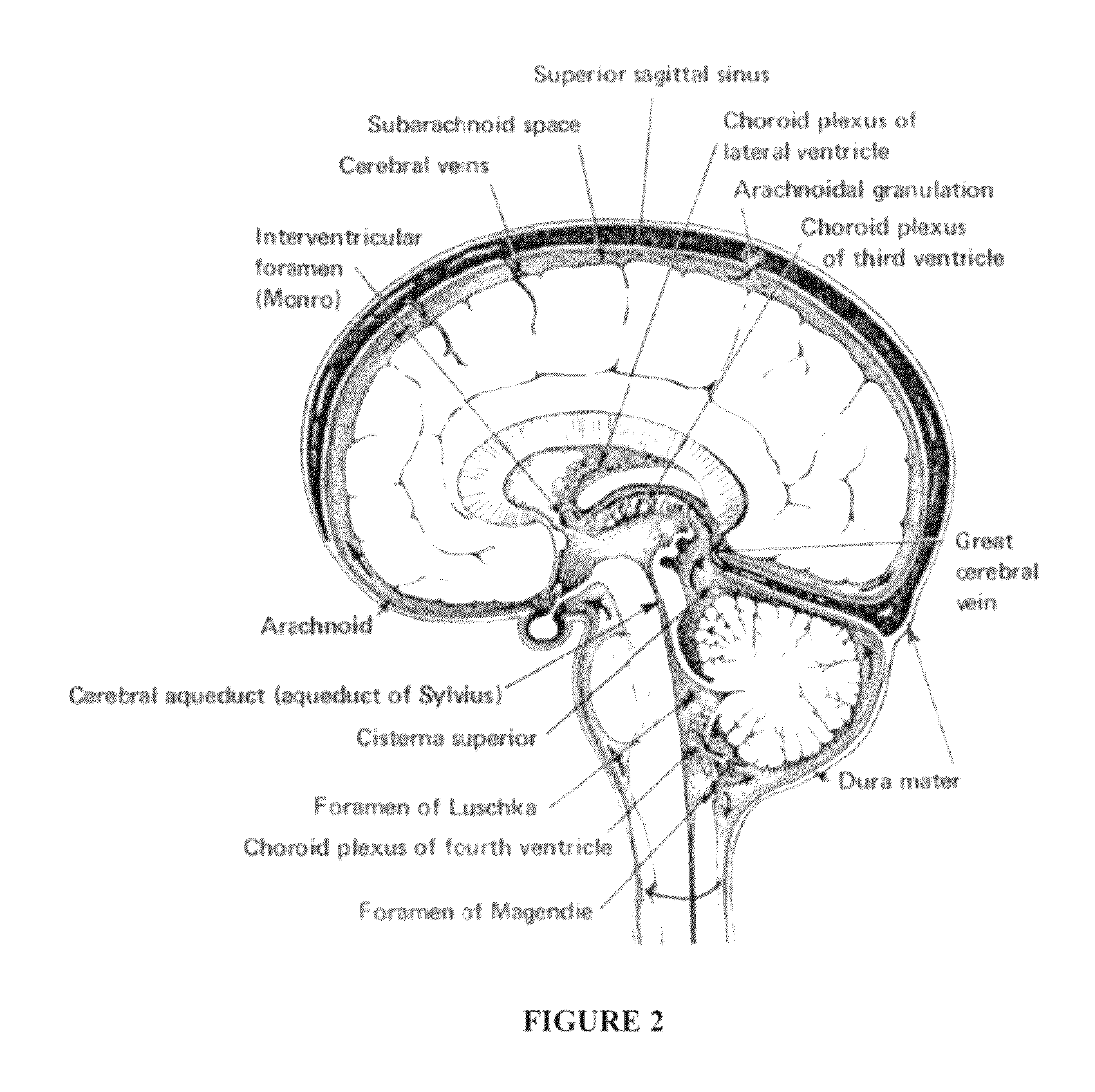
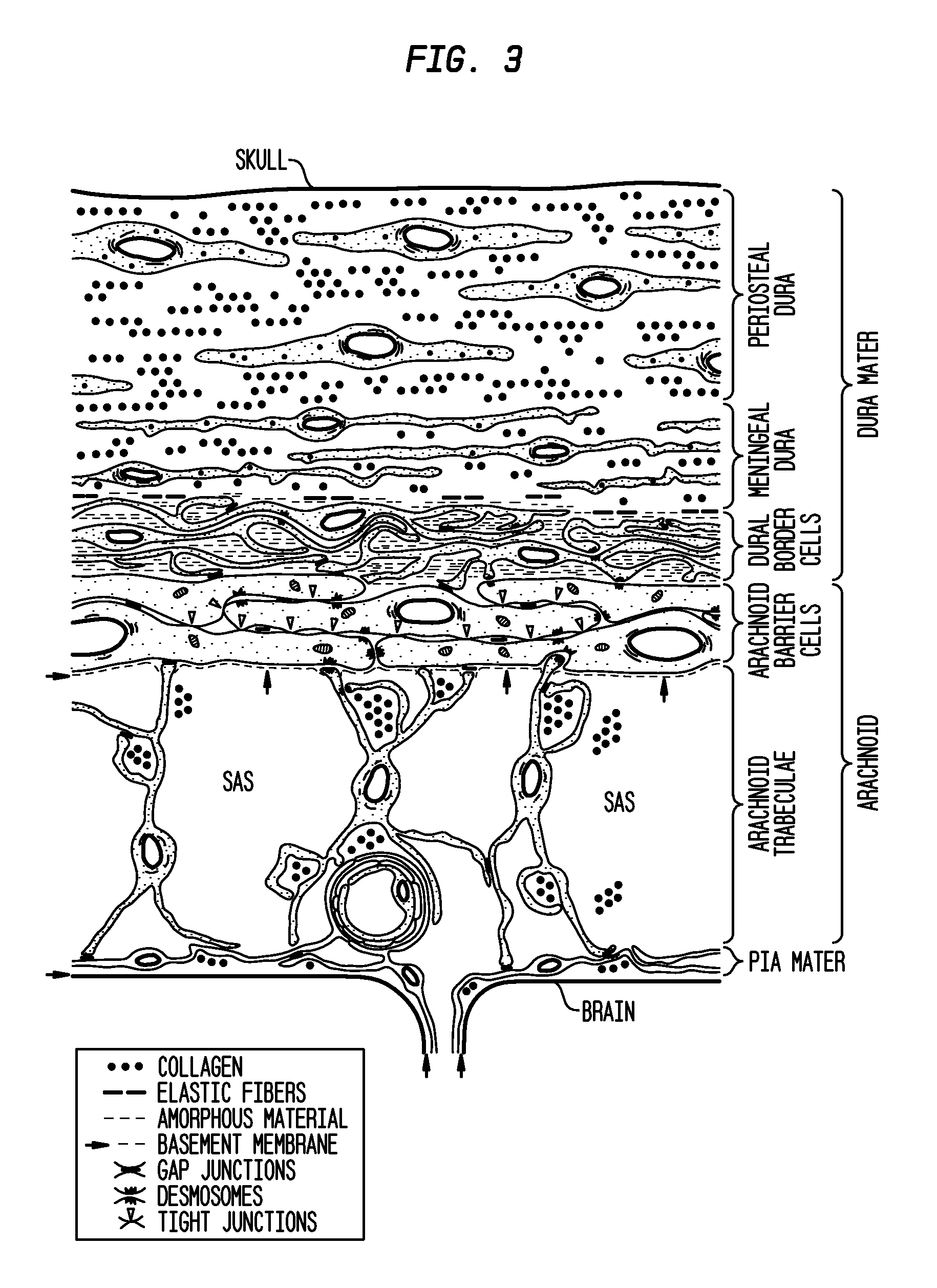
Compositions and their use to treat complications of aneurysmal subarachnoid hemmorrhage
Owner:EDGE THERAPEUTICS
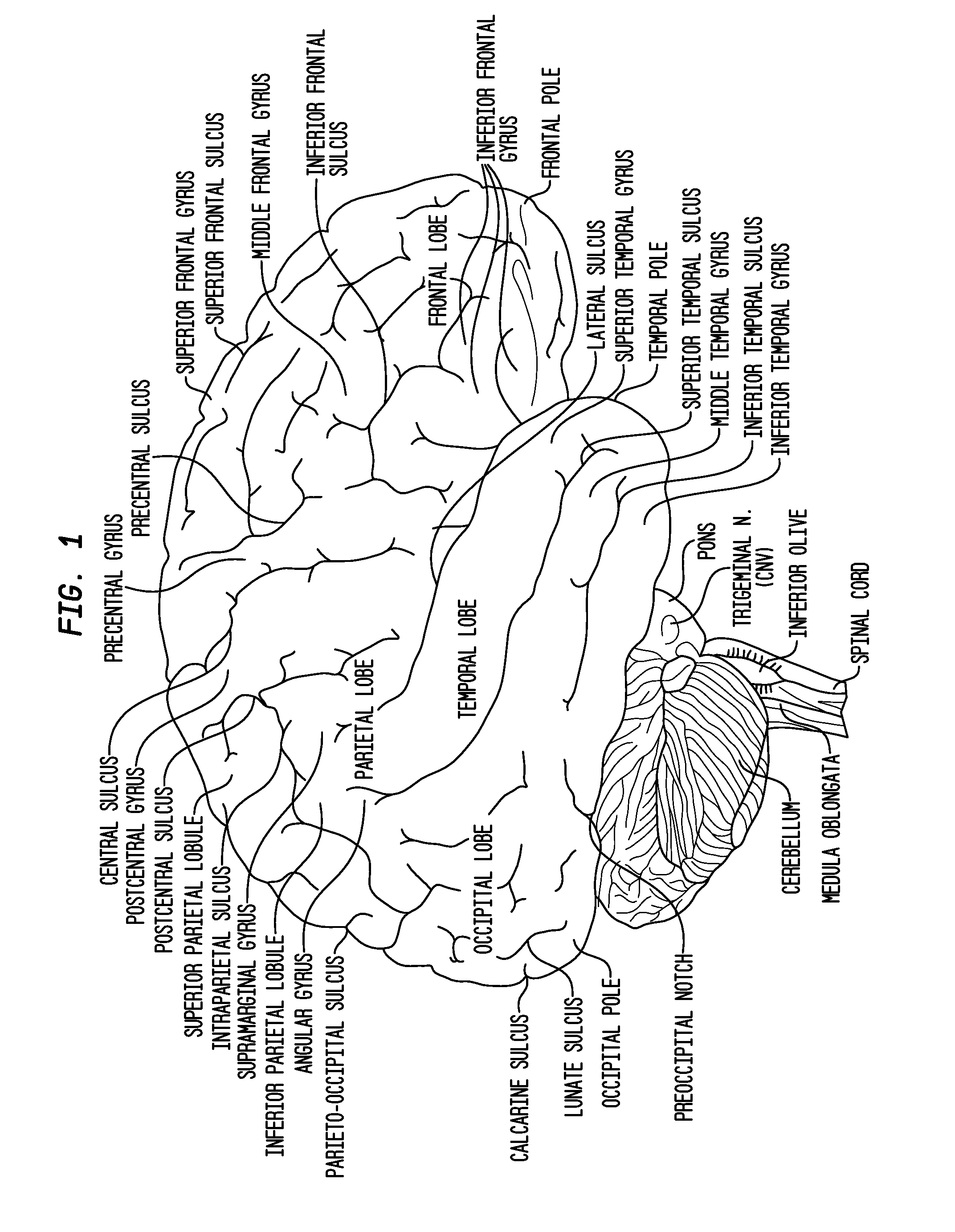
Intraventricular drug delivery system for improving outcome after a brain injury affecting cerebral blood flow
The described invention provides a flowable sustained release microparticulate composition, a kit for treating at least one cerebral artery in a subarachnoid space at risk of interruption due to a brain injury, a method of preparing the composition, and a method for treating an interruption of a cerebral artery in a subarachnoid space at risk of interruption caused by brain injury in a mammal, which reduces signs or symptoms of at least one delayed complication associated with brain injury.
Owner:EDGE THERAPEUTICS
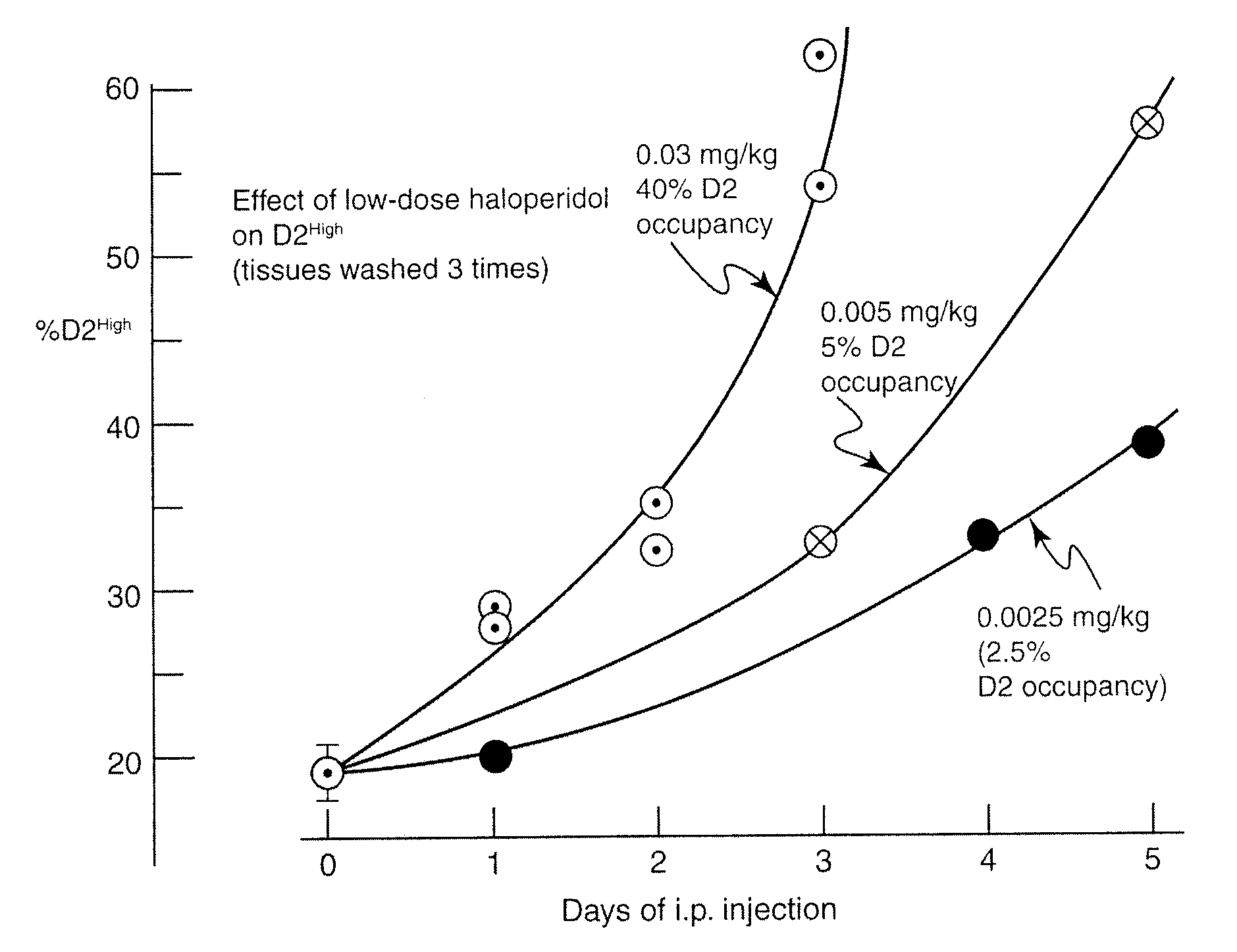
Compositions and methods for treating parkinson's disease
InactiveUS9192605B2Relieve symptomsImprove the level ofBiocideNervous disorderTypical antipsychoticTrifluperidol
This application describes compositions of receptor inhibitors, including typical antipsychotic agents, for example haloperidol, and methods of use for treating clinical signs or symptoms of Parkinson's disease. Furthermore, this application describes compositions and methods to induce supersensitivity in dopamine D2 and other receptors involved in Parkinson's disease as a means of alleviating the clinical signs or symptoms of Parkinson's disease.
Owner:CLERA INC
ON01910.Na enhances chemotherapeutic agent activity in drug-resistant tumors
The invention includes compositions and methods of treatment of cancers susceptible to treatment with nucleotide analog chemotherapeutic agent, including cancers in which nucleotide analog resistant tumors have developed, including identifying a subject having cancer susceptible to treatment with a nucleotide analog chemotherapeutic agent and a mitotic disruptor / polo-like kinase (Plk) pathway inhibitor to a subject; and monitoring the subject for a reduction or stabilization of at least one sign or symptom of cancer.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
Formulations of site-specific, microparticulate compositions and their use to improve outcome after aneursymal subarachnoid hemorrhage
The described invention provides site-specific sustained release microparticulate formulations containing a therapeutic amount of an L-type voltage gated calcium channel inhibitor, a PLGA polymer comprising from 25% to 50% glycolide, and a hyaluronic acid. A therapeutic amount of the formulation is effective to reduce signs or symptoms of delayed cerebral ischemia comprising one or more of a cortical spreading ischemia, a cortical spreading depolarization, a plurality of microthromboemboli, or an angiographic vasospasm after brain injury in a mammal, while reducing the risk of systemic hypotension, cardiac dysfunction, anoxia, and intracranial hypertension.
Owner:EDGE THERAPEUTICS
Kynurenine-3-monooxygenase inhibitors, pharmaceutical compositions, and methods of use thereof
Certain compounds, or pharmaceutically acceptable salts or prodrugs thereof, pharmaceutical compositions comprising the same and methods of treating patients suffering from certain diseases and disorders responsive to the inhibition of KMO activity are described, which comprise administering to such patients an amount of at least one compound, or pharmaceutically acceptable salt or prodrug thereof described herein, effective to reduce signs or symptoms of the disease or disorder. These diseases include neurodegenerative disorders such as Huntington's disease. The application also provides a method for screening a compound capable of inhibiting KMO activity.
Owner:CHDI FOUND
Intraventricular drug delivery system for improving outcome after a brain injury affecting cerebral blood flow
The described invention provides a flowable sustained release microparticulate composition, a kit for treating at least one cerebral artery in a subarachnoid space at risk of interruption due to a brain injury, a method of preparing the composition, and a method for treating an interruption of a cerebral artery in a subarachnoid space at risk of interruption caused by brain injury in a mammal, which reduces signs or symptoms of at least one delayed complication associated with brain injury.
Owner:EDGE THERAPEUTICS
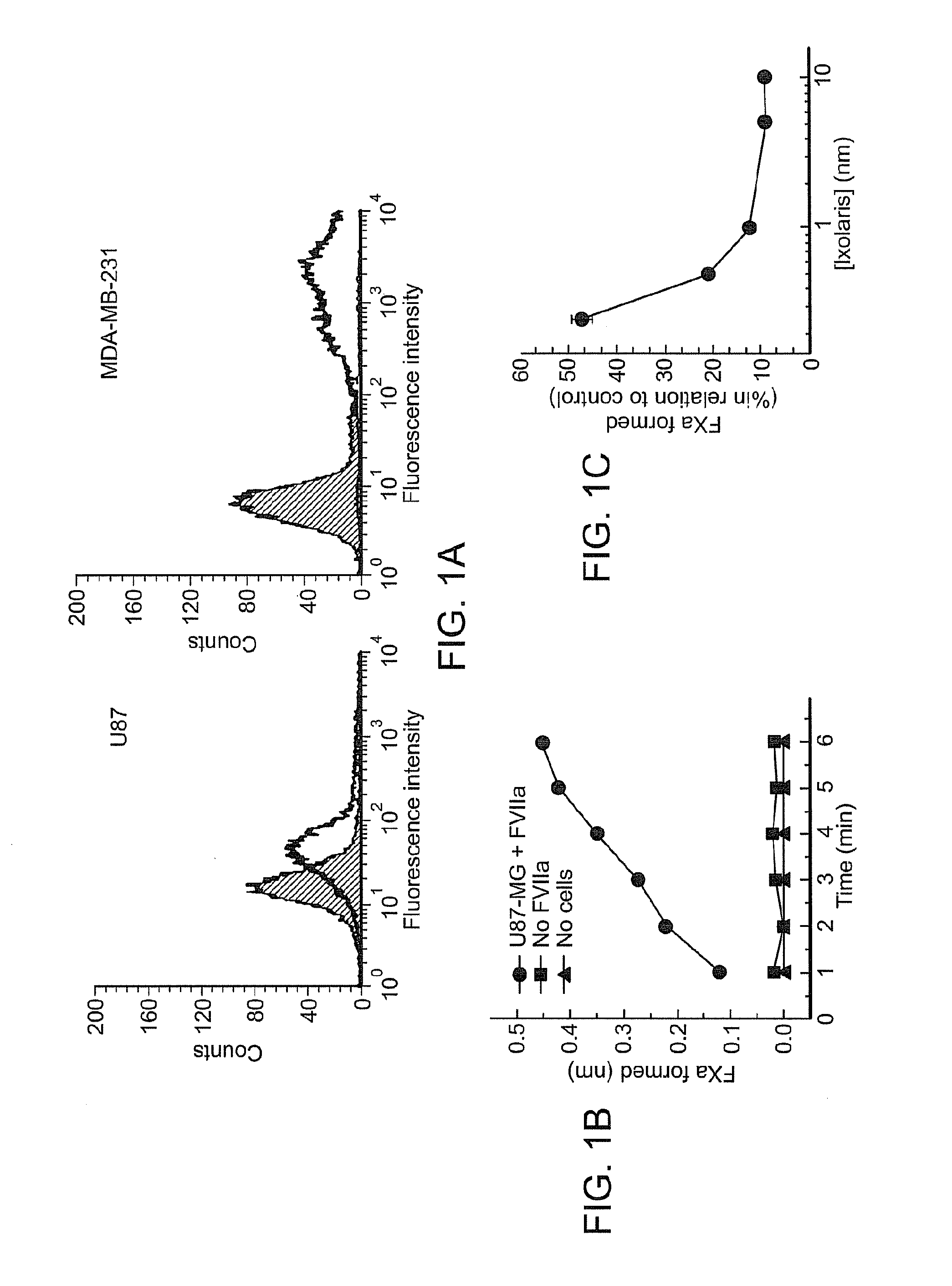
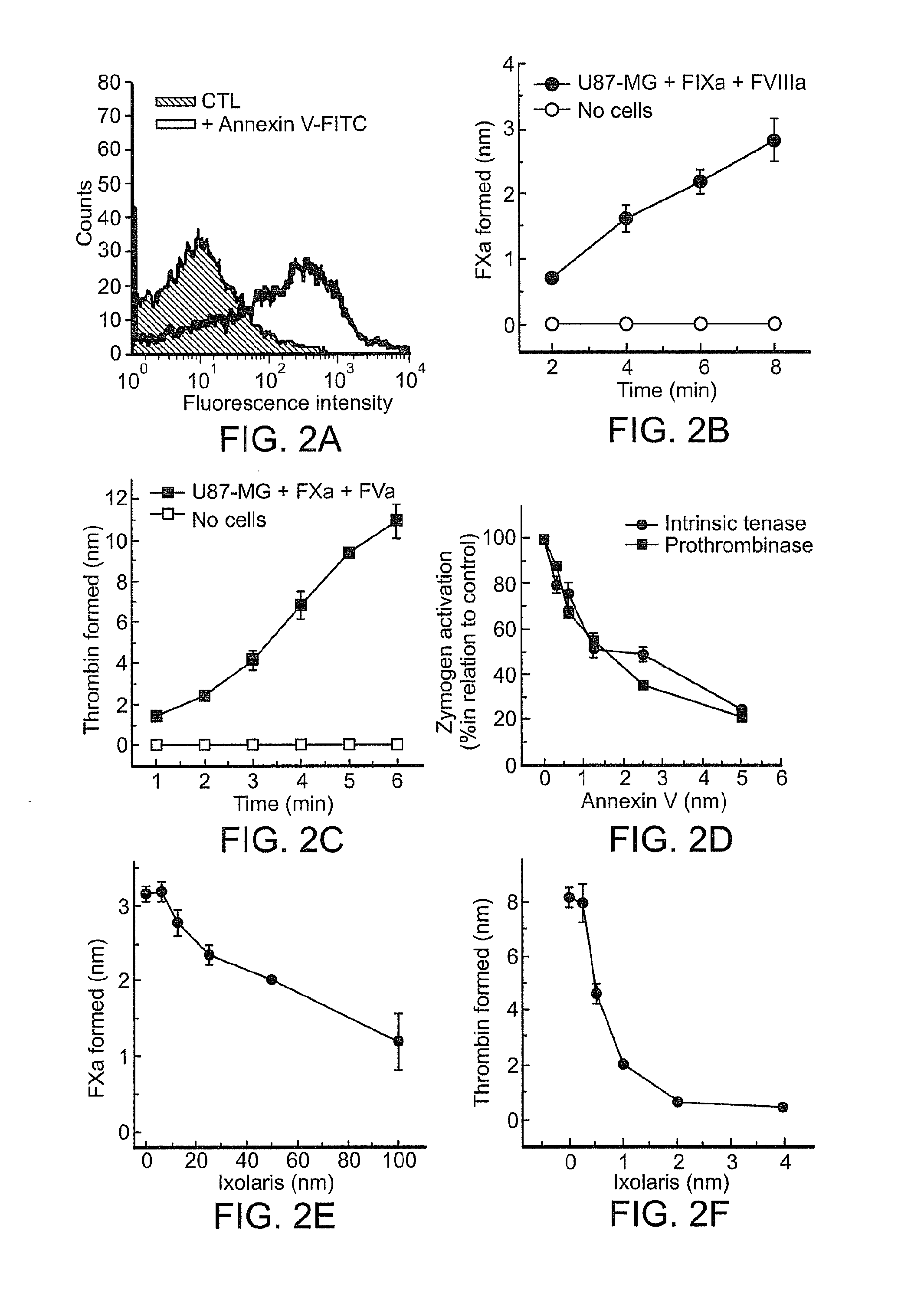
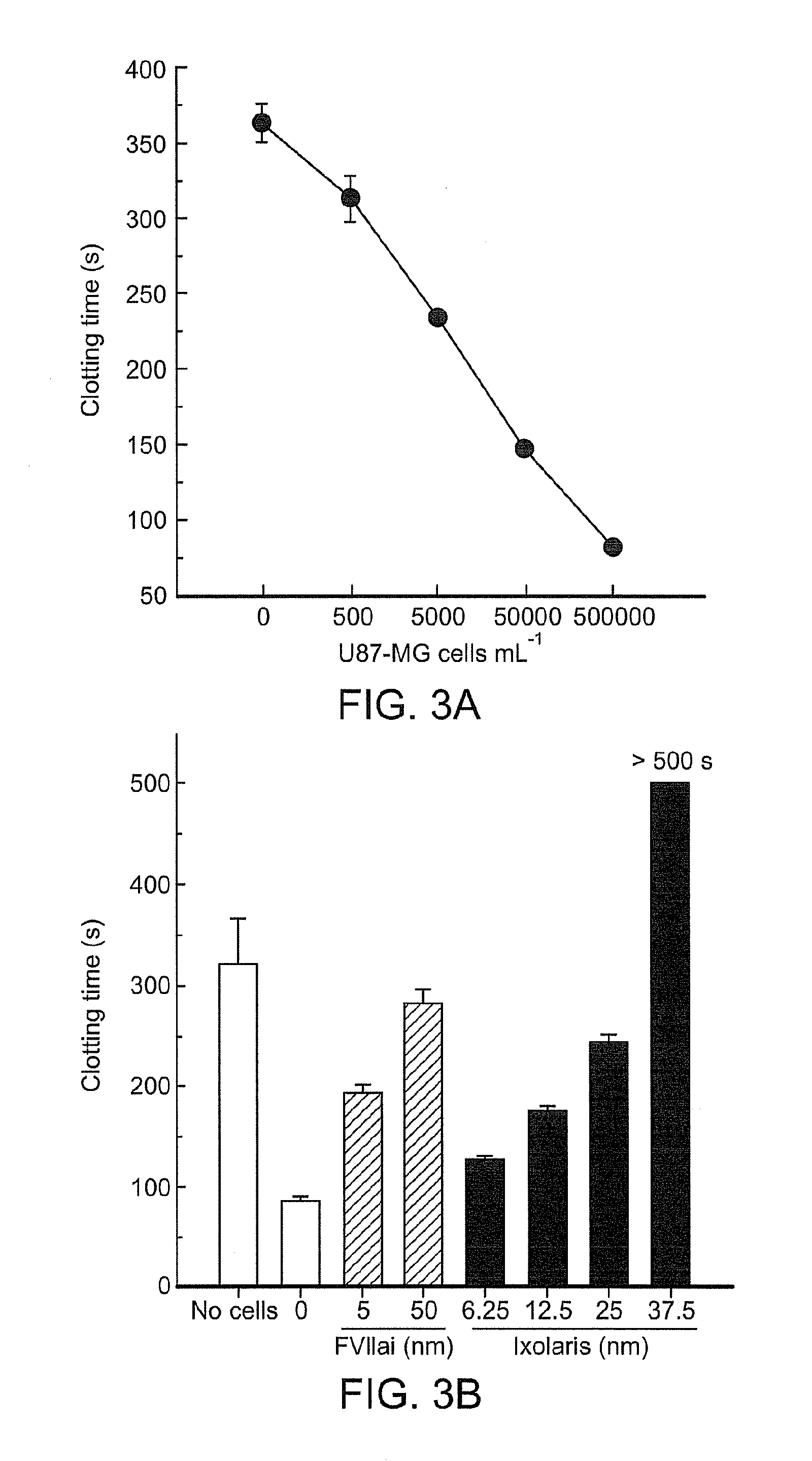
Use of ixolaris, a tissue factor inhibitor, for the treatment and prevention of cancer
ActiveUS20120010137A1Reduce rateStop tumor growthSenses disorderPeptide/protein ingredientsTissue factorCancer prevention
The invention provides methods for treatment of tissue factor (TF) mediated or associated diseases or processes, such as cancer, by administering at least an active fragment of an Ixolaris polypeptide to a subject. The invention further includes identification of a subject in need of such treatment, and monitoring a subject for amelioration of at least one sign or symptom of the disease. The invention also features kits.
Owner:UNITED STATES OF AMERICA
Compositions and methods for treating smooth muscle dysfunction
InactiveUS20160184455A1Less heightened contractilityCell receptors/surface-antigens/surface-determinantsSugar derivativesDiseasePotassium
The present disclosure provides compositions and methods to improve one or more signs or symptoms of smooth muscle diseases. Compositions of the disclosure may include a plasmid vector containing a variant nucleic that encodes for a variant amino acid sequence of the alpha subunit of the BK potassium channel. Compositions may further include a nanoparticle delivery system. Compositions and methods of use of the disclosure may be used to treat, for example, over active bladder (OAB) syndrome and erectile dysfunction (ED).
Owner:ION CHANNEL INNOVATIONS +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
![Certain heterocyclic substituted imidazo[1,2-A]pyrazin-8-ylamines and methods of inhibition of Bruton's tyrosine kinase by such compounds Certain heterocyclic substituted imidazo[1,2-A]pyrazin-8-ylamines and methods of inhibition of Bruton's tyrosine kinase by such compounds](https://images-eureka.patsnap.com/patent_img/ebbaacdc-083b-4261-bc4a-f1b6b1a1c3a3/US07393848-20080701-C00001.png)
![Certain heterocyclic substituted imidazo[1,2-A]pyrazin-8-ylamines and methods of inhibition of Bruton's tyrosine kinase by such compounds Certain heterocyclic substituted imidazo[1,2-A]pyrazin-8-ylamines and methods of inhibition of Bruton's tyrosine kinase by such compounds](https://images-eureka.patsnap.com/patent_img/ebbaacdc-083b-4261-bc4a-f1b6b1a1c3a3/US07393848-20080701-C00002.png)
![Certain heterocyclic substituted imidazo[1,2-A]pyrazin-8-ylamines and methods of inhibition of Bruton's tyrosine kinase by such compounds Certain heterocyclic substituted imidazo[1,2-A]pyrazin-8-ylamines and methods of inhibition of Bruton's tyrosine kinase by such compounds](https://images-eureka.patsnap.com/patent_img/ebbaacdc-083b-4261-bc4a-f1b6b1a1c3a3/US07393848-20080701-C00003.png)