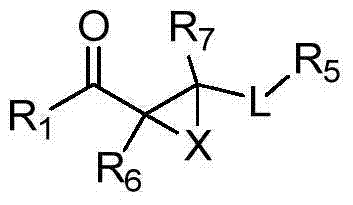
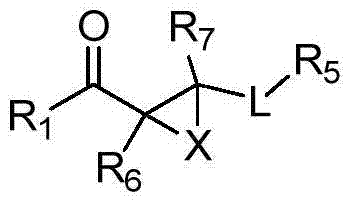
Kynurenine-3-monooxygenase inhibitors, pharmaceutical compositions, and methods of use thereof
A technology of medicinal salts and compounds, applied in the field of kynurenine-3-monooxygenase inhibitors
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
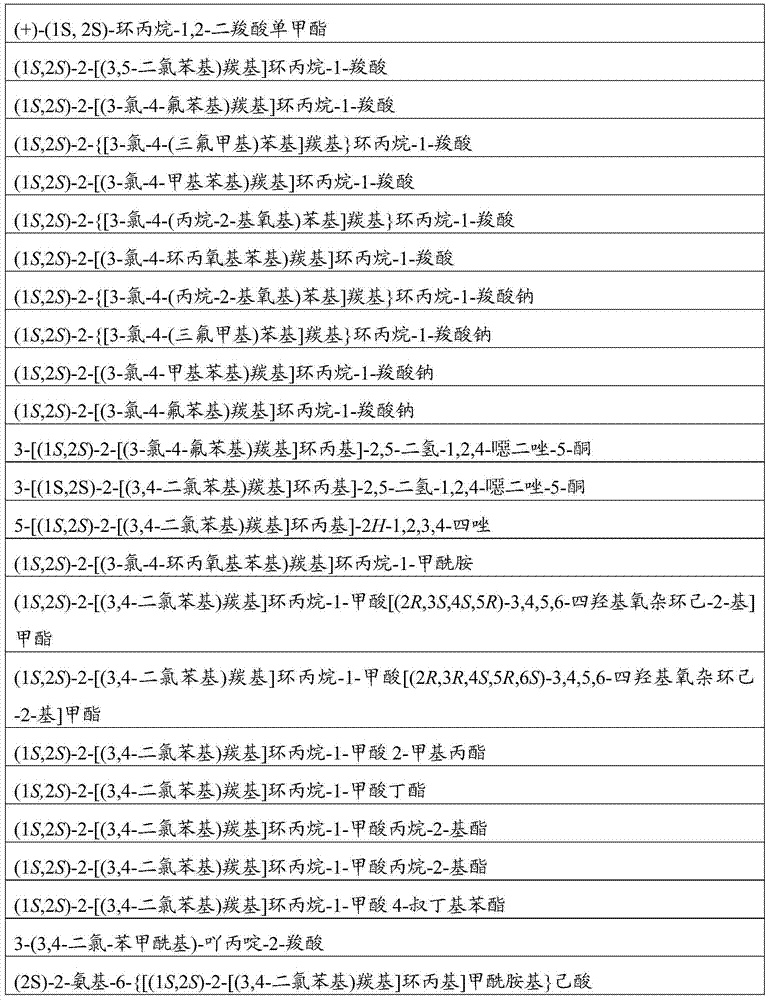
[0277] (+)-(1S,2S)-Cyclopropane-1,2-dicarboxylic acid monomethyl ester prepared as described in EP1475385, 2004
[0278]
[0279] Step 1, Method 1: Methyl (1S,2S)-2-(chlorocarbonyl)cyclopropane-1-carboxylate
[0280] DMF (0.16 mL, 2.08 mmol) was added dropwise to (+)-(1S,2S)-cyclopropane-1,2-dicarboxylic acid monomethyl ester (3.0 g, 20.8 mmol) under nitrogen atmosphere at room temperature A stirred solution in DCM (80 mL). Oxalyl chloride (5.45 mL, 62.4 mmol) was added dropwise to the reaction mixture over 30 minutes, and the reaction was stirred at room temperature for 1 hour. The reaction mixture was then concentrated and the resulting residue was co-evaporated with DCM (3 x 20 mL) to give the title compound (3.42 g, 98% yield) as a yellow oil which was used without further purification.
[0281] Step 2, Method 1: Methyl (1S,2S)-2-[(3,4-dichlorophenyl)carbonyl]cyclopropane-1-carboxylate
[0282] See eg, Naoaki et al, Bioorganic and Medicinal Chemistry Letters, 2005, 1...
Embodiment 2
[0307]
[0308] Step 1, 4-Bromo-2-chloro-1-cyclopropoxybenzene
[0309] Add bromocyclopropane (14.6g, 24.0mmol) in one portion to 4-bromo-2-chlorophenol (5.0g, 24.0mmol) and cesium bicarbonate (19.6g, 60.0mmol) in dimethylacetamide (80mL) in the stirred solution. The mixture was heated to 150°C and stirred at this temperature for 16 hours. Then, another portion of bromocyclopropane (14.6 g, 24.0 mmol) was added, and the mixture was stirred at 150° C. for another 24 hours. The reaction mixture was then cooled to room temperature, poured onto ice-water (200 mL), extracted with TBME (3 x 200 mL). The combined organic layers were washed with water (2 x 100 mL) followed by brine (50 mL). The organic layer was removed and dried (MgSO 4 ), filtered and concentrated. The resulting residue was purified by flash column chromatography (elution: heptane, then 33% heptane, 67% DCM) to afford the title compound (5.0 g, 84% yield) as a colorless oil. Tr=2.41min m / z (ES + )(M+H + )...
Embodiment 3
[0321]
[0322] Step 1, Sodium (1S,2S)-2-{[3-chloro-4-(propan-2-yloxy)phenyl]carbonyl}cyclopropane-1-carboxylate
[0323] (1S,2S)-2-{[3-Chloro-4-(propan-2-yloxy)phenyl]carbonyl}cyclopropane-1-carboxylic acid (0.14g, 0.51mmol) in NaOH (2M solution , 0.23mL, 0.46mmol) and stirred for 1 hour. Then, diethyl ether (2 mL) was added, and the organic layer was separated and discarded. The aqueous layer was concentrated to afford the title compound (0.097 g, 62% yield) as an off-white solid.
[0324] Sodium (1S,2S)-2-{[3-chloro-4-(propan-2-yloxy)phenyl]carbonyl}cyclopropane-1-carboxylate
[0325] δ H (500MHz,DMSO)7.88-7.97(2H,m)7.30(1H,d)4.78-4.89(1H,m)2.77-2.89(1H,m)1.64-1.74(1H,m)1.33(6H,d)1.14 -1.25(2H,m). Tr=4.03min m / z (ES + )(M+H + )283,285.
[0326] The following compounds were prepared essentially as described above.
[0327] Sodium (1S,2S)-2-{[3-chloro-4-(trifluoromethyl)phenyl]carbonyl}cyclopropane-1-carboxylate
[0328] δ H (500MHz, DMSO) 7.99-8.16 (3H, m) 2.86...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com