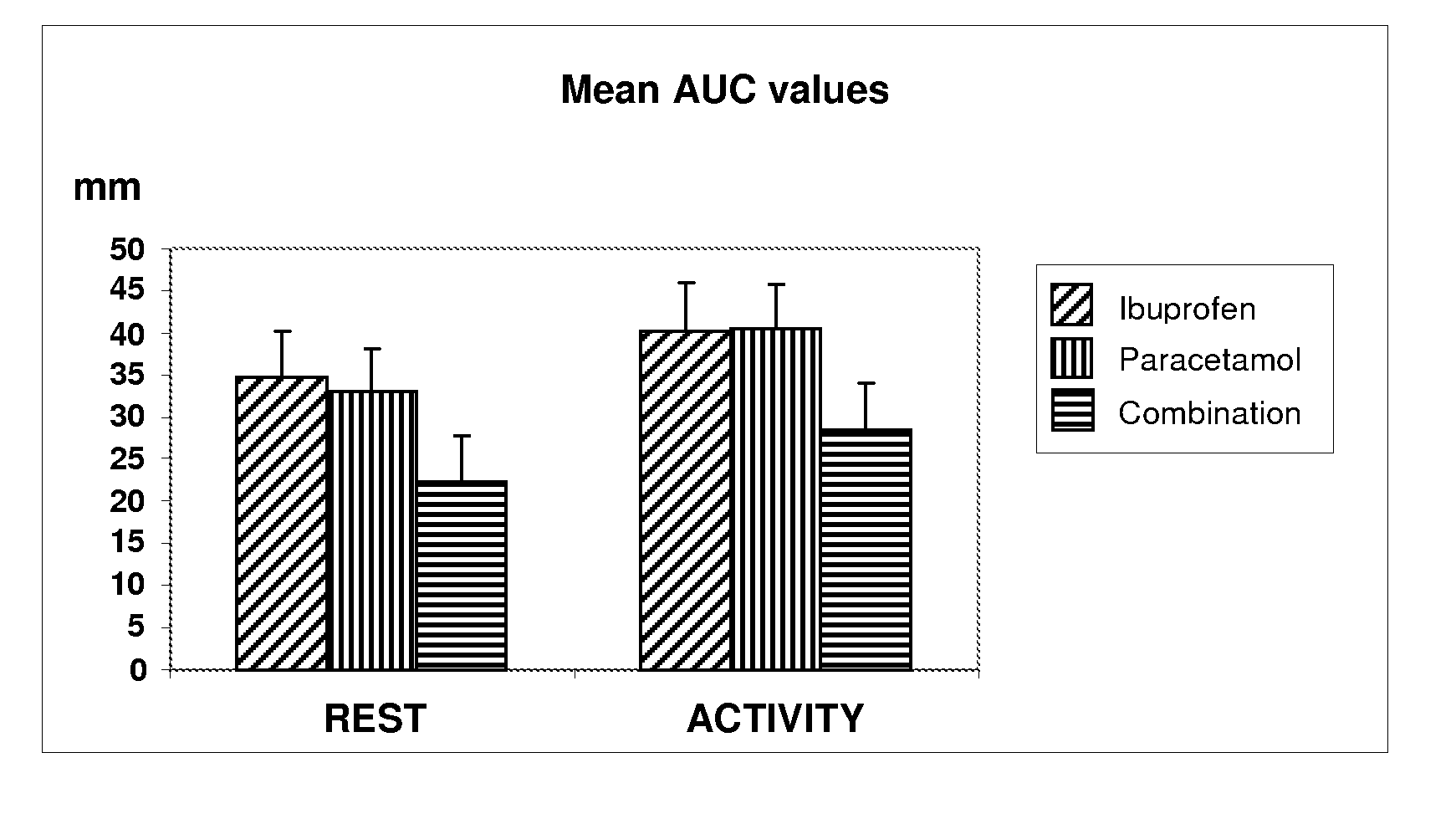
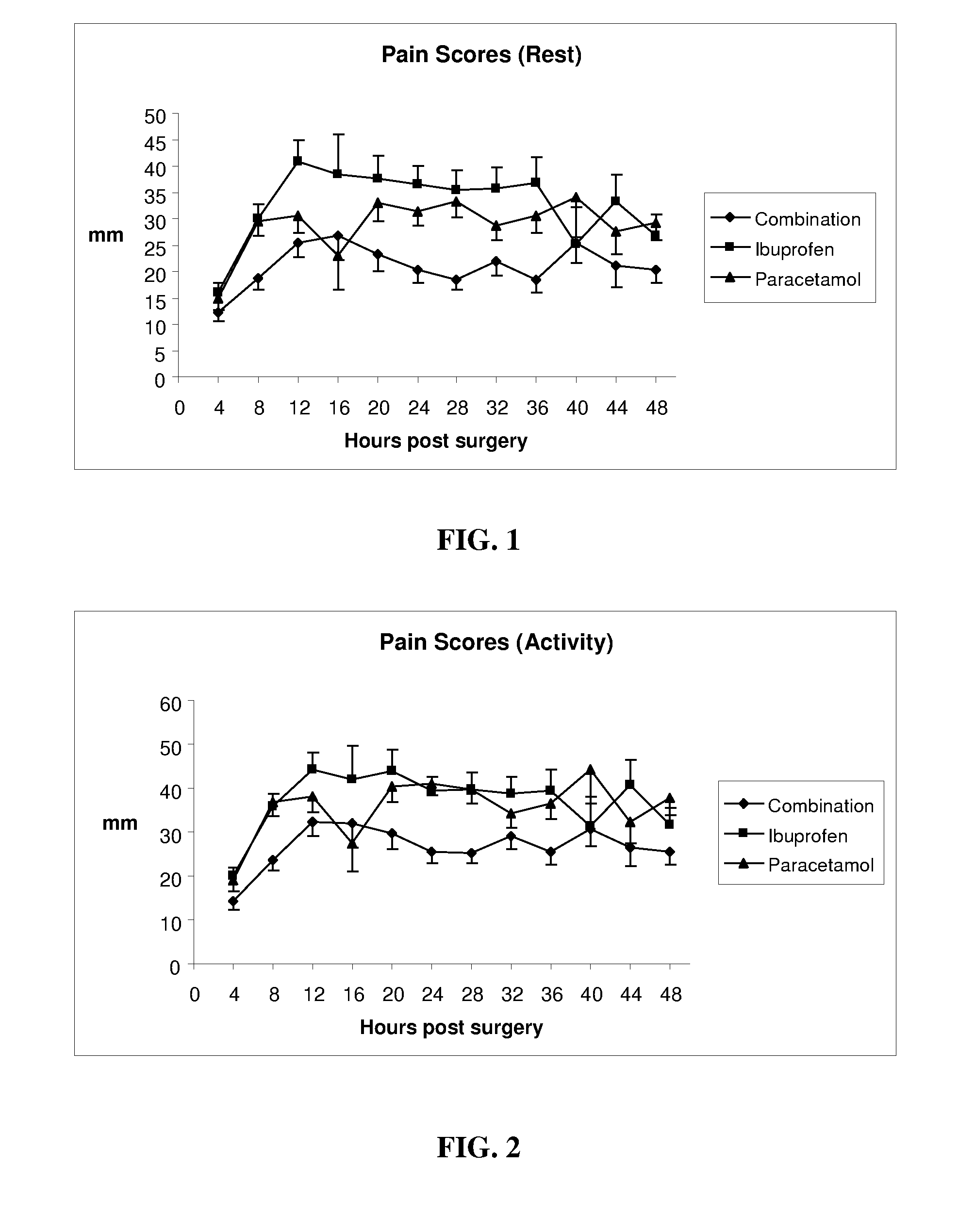
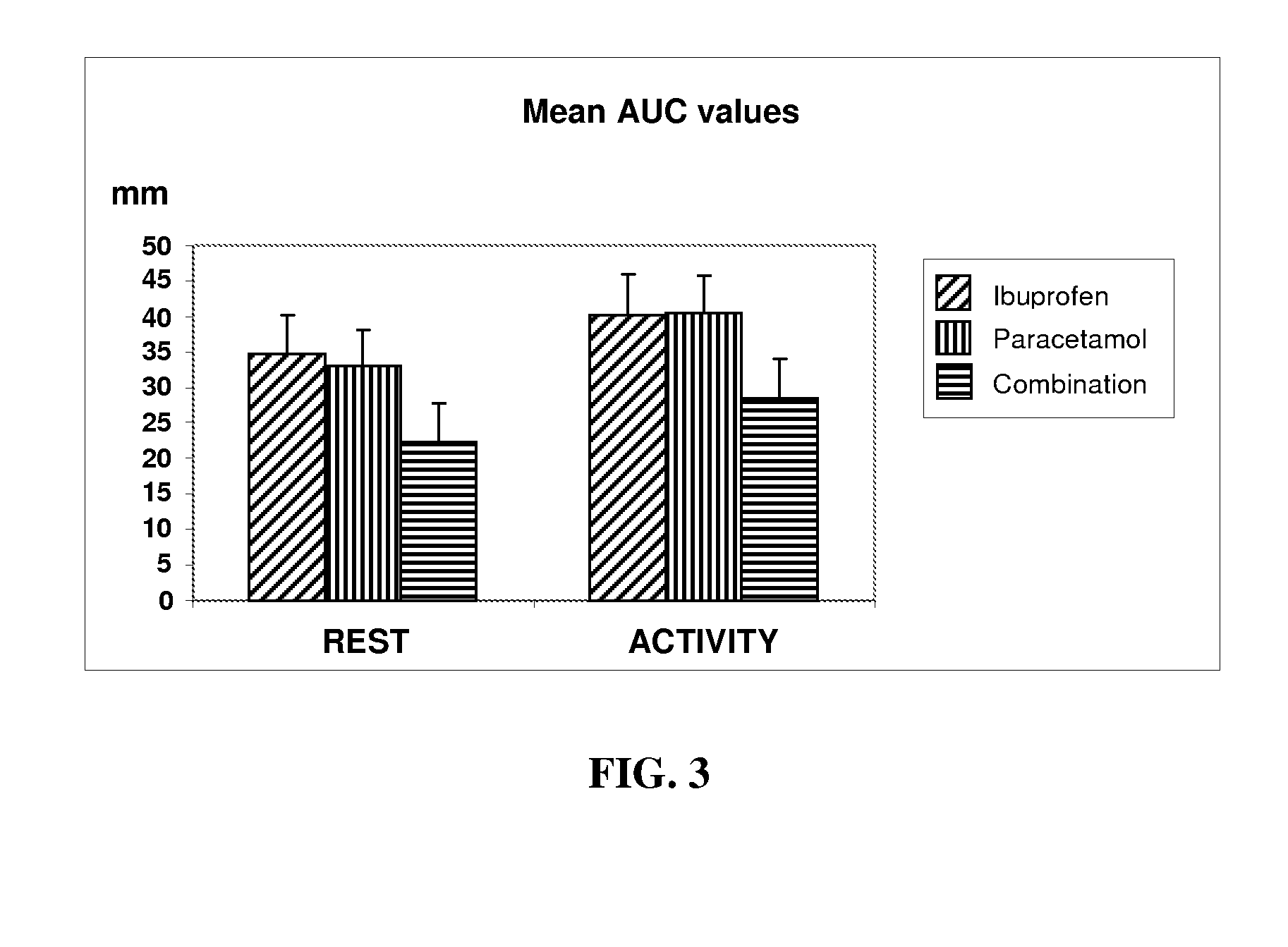
Pharmaceutical composition of ibuprofen and paracetamol and methods of using the same
a technology of ibuprofen and paracetamol, which is applied in the field of combination compositions for pain treatment, can solve the problems of reducing the efficacy of pain relief, still associated with side effects in some individuals, and affecting the health of patients,
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
Tablets for Oral Use
[0097]
Core:Paracetamol500.0mgIbuprofen150.0mgMaize Starch (dry mix)14.83mgColloidal Silicon Dioxide1.70mgMaize Starch (for paste)22.5mgDisodium EDTA0.50mgPolyvinyl Pyrrolidone7.54mgSodium Benzoate1.00mgMaize Starch (Lubrication)12.50mgColloidal Silicon Dioxide12.00mgMagnesium Stearate2.45mgSodium Starch Glycolate25.00mgPurified Water q.s.Coating:Hydroxypropylmethyl Cellulose7.20mgPolyethylene Glycol 60000.80mgTitanium Dioxide (Colorant)0.21mgMethylhydroxybenzoate0.20mgPropylhydroxybenzoate0.02mgPurified Water q.s.Total:758.45mg
example 2
Preparation of Tablets
[0098]A Preparation of a Granulation Mixture
[0099]1. Weigh paracetamol and ibuprofen and sift using a suitable vibrosifter and transfer to mixer. Discard any material not passing through #12 sieve.
[0100]2. Weigh and sift maize starch using a suitable vibrosifter (#40 sieve) and transfer to mixer.
[0101]3. Weigh and sift colloidal silicon dioxide using a suitable vibrosifter (#100 sieve) and transfer to mixer.
[0102]4. Mix for 10-11 minutes at slow speed.
[0103]B. Wet Granulation and Drying
[0104]1. Add purified water (0.03 ml / tablet) to stainless steel container
[0105]2. Sift maize starch (for paste) using suitable sieve (for example #60) and stir until slurry is formed.
[0106]3. Add purified water (0.18 ml / tablet) to a suitable jacketed planetary mixer and heat to boiling.
[0107]4. Add disodium EDTA, polyvinyl pyrrolidine and sodium benzoate. Dissolve and stir for 5-6 minutes until a clear solution is obtained.
[0108]5. Add starch slurry under continuous stirring unti...
example 3
Tablets for Oral Use
[0122]
Part I (Dry Mixing)Paracetamol500.0 mgIbuprofen150.0 mgMaize Starch (dry mix)25.32 mgMicrocrystalline Cellulose30.00 mgPregelatanized starch32.00 mgCroscarmellose sodium 2.50 mgPart II (Granulation)Maize starch (paste)50.00 mgMethyl parahydoxybenzoate 0.30 mgPropyl parahydroxybenzoate 0.03 mgPurified Water q.s.Part III (Lubrication)Maize starch10.00 mgCroscarmellose sodium10.00 mgMagnesium stearate 4.85 mgTalc10.00 mgFilm CoatingOpadry white OYLS 5890014.00 mgTalc 1.00 mgPurified Water q.s.
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight ratio | aaaaa | aaaaa |
| weight ratio | aaaaa | aaaaa |
| weight ratio | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com