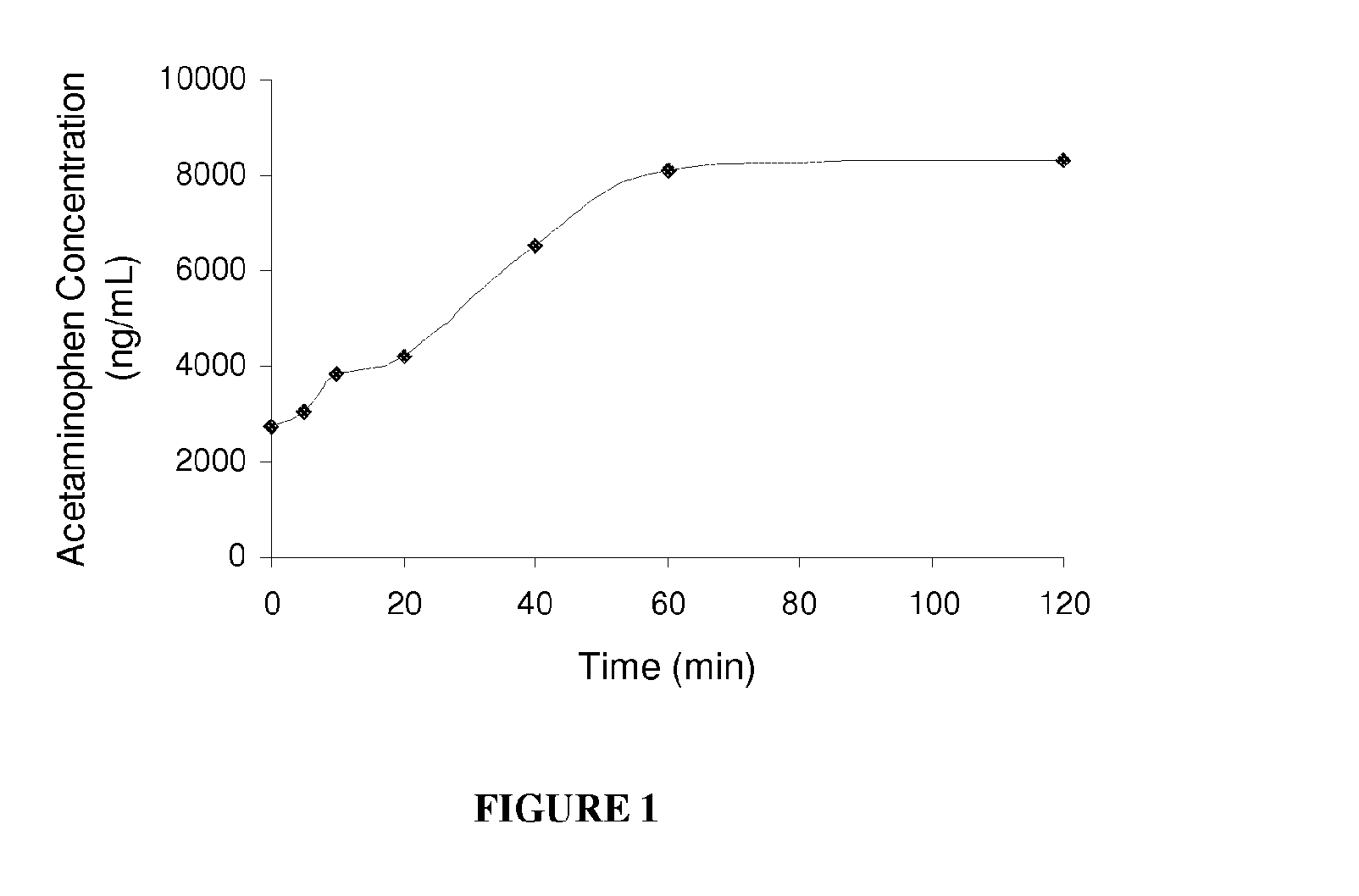
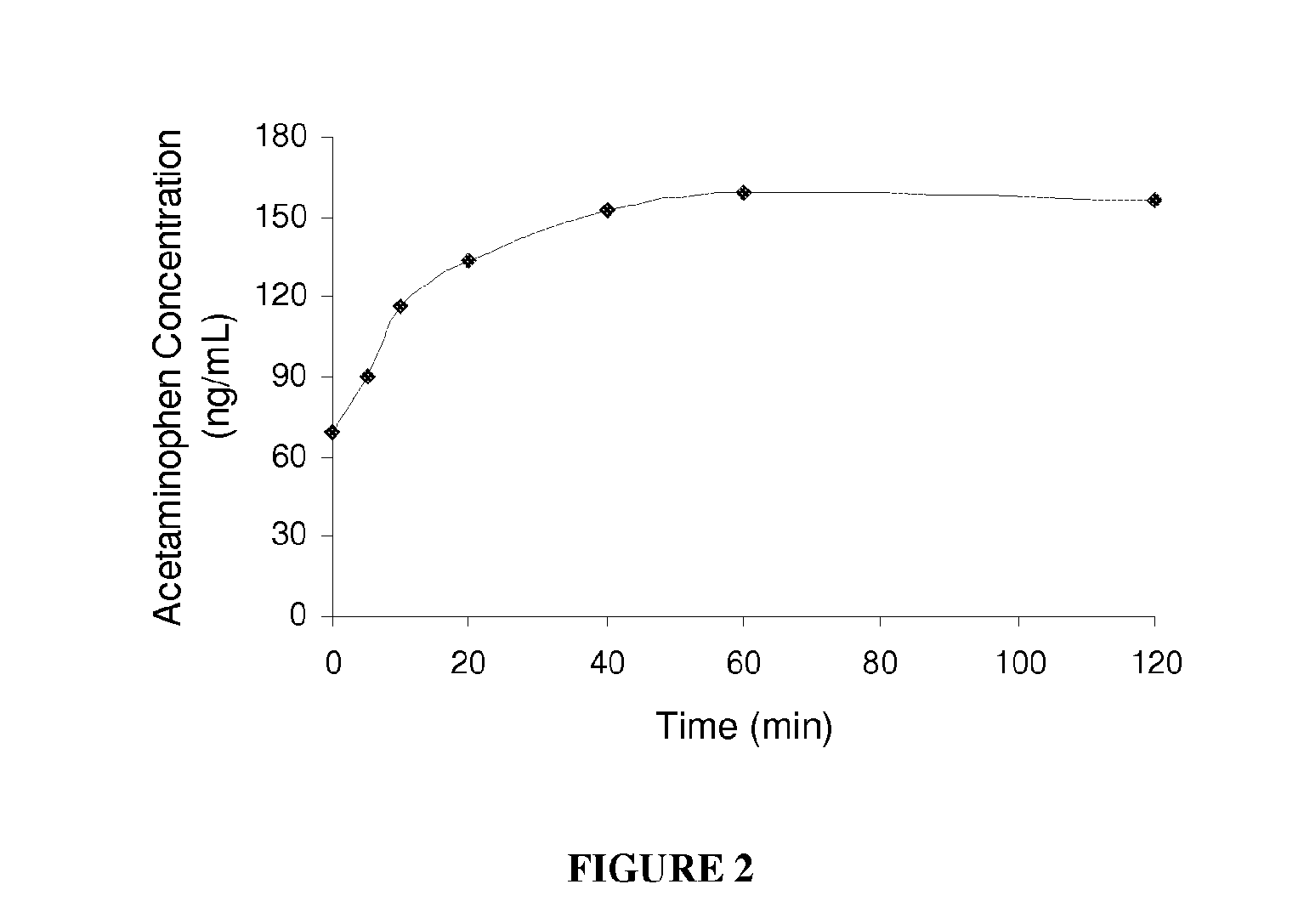
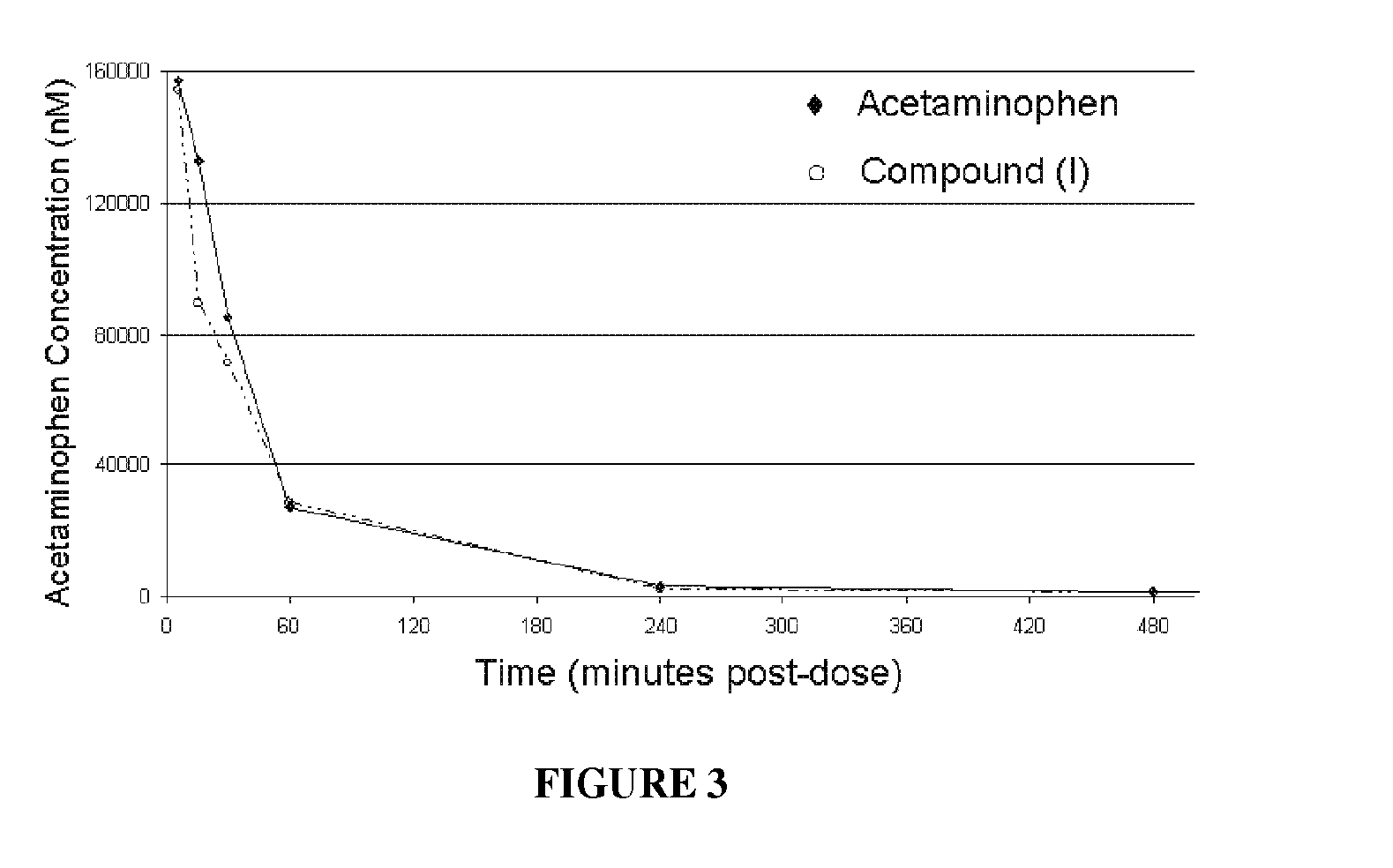
Water-soluble acetaminophen analogs
a technology of acetaminophen and analogs, applied in the field of water-soluble acetaminophen analogs, can solve the problems of affecting and affecting the development of effective therapeutic acetaminophen beyond oral dosage forms, so as to delay the onset of acetaminophen action and slow the onset of acetaminophen
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Synthesis of (4-acetamidophenoxy)methyl dihydrogen phosphate
N-(4-(methylthiomethoxy)phenyl)acetamide
[0106]
[0107]To a stirred ash-colored suspension of sodium hydride (1.9 g; 75.49 mmol) in hexamethylphosphoramide (HMPA; 25 mL) cooled to 0° C., a solution of acetaminophen (10 g; 66.22 mmol in 35 mL of HMPA) was added slowly in about 15 minutes. The ash-colored suspension changed to a light-brown clear solution after 15 minutes of stirring. The reaction mixture was stirred for an additional 20 minutes at 0° C. Then chloromethyl methyl sulfide (7.67 g; 79.47 mmol) was slowly added to the reaction mixture over about 10 minutes. There was no color change in reaction mixture. The reaction mixture was slowly warmed to room temperature and stirring continued for 3 hr. Rf values of starting material and product, in a methanol:dichloromethane solvent system (3:97) on TLC silica gel 60 F254 (Merck) detected at λ 254 nm, were 0.4, and 0.6, respectively. The reaction mixture was quenched with sa...
example 2
Synthesis of Sodium (4-acetamidophenoxy)methyl phosphate
[0112]
[0113]To a stirring milky suspension of (4-acetamidophenoxy)methyl dihydrogen phosphate (20 mg; 0.076 mmol) in ethyl acetate (5 mL), a solution of sodium-2-ethyl hexanoate (25 mg; 0.15 mmol) in ethyl acetate (2 mL) was added. The reaction suspension was stirred for 3 h. The milky suspension turned to a white solid after one hour, which was filtered, washed with ethyl acetate (3×15 mL) and ether (3×20 mL) to yield 10 mg (50% yield) of product as a white solid (melting range: 206-209° C.). 1H NMR (400 MHz, DMSO): δ 9.8 (s, 1H), 7.41 (d, 2H, J=10.8 Hz), 6.96 (d, 2H, J=11.6 Hz), 5.26 (d, 2H, J=10.4 Hz), 1.97 (s, 3H).
example 3
Solubility of Acetaminophen Analogs
[0114]Solubility of (4-acetamidophenoxy)methyl dihydrogen phosphate and its disodium salt is listed in Table. 1.
TABLE 1Solubility of Acetaminophen ProdrugsSolubilitySoluteSolvent(mg / mL)(4-Water145acetamidophenoxy)methyldihydrogen phosphateSodium (4-Water160acetamidophenoxy)methylphosphate
PUM
| Property | Measurement | Unit |
|---|---|---|
| volume | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com