Method for preparing compound paracetamol and amantadine hydrochloride tablet
A technology for acetaminophen and amantadine hydrochloride, which is applied in pharmaceutical formulas, active ingredients of amines, medical preparations containing active ingredients, etc., and can solve the problem that excipients are not allowed to be replaced casually
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
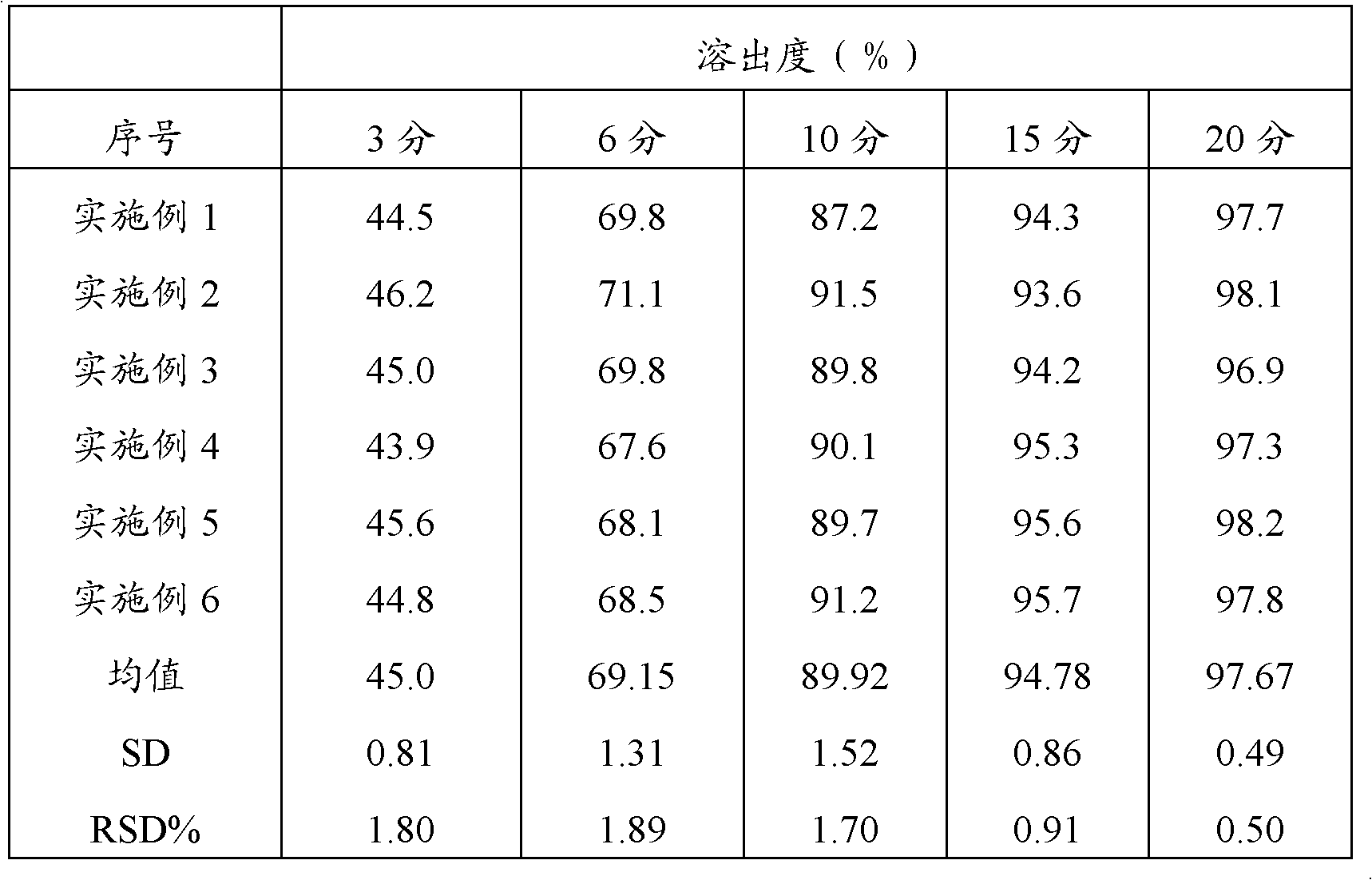
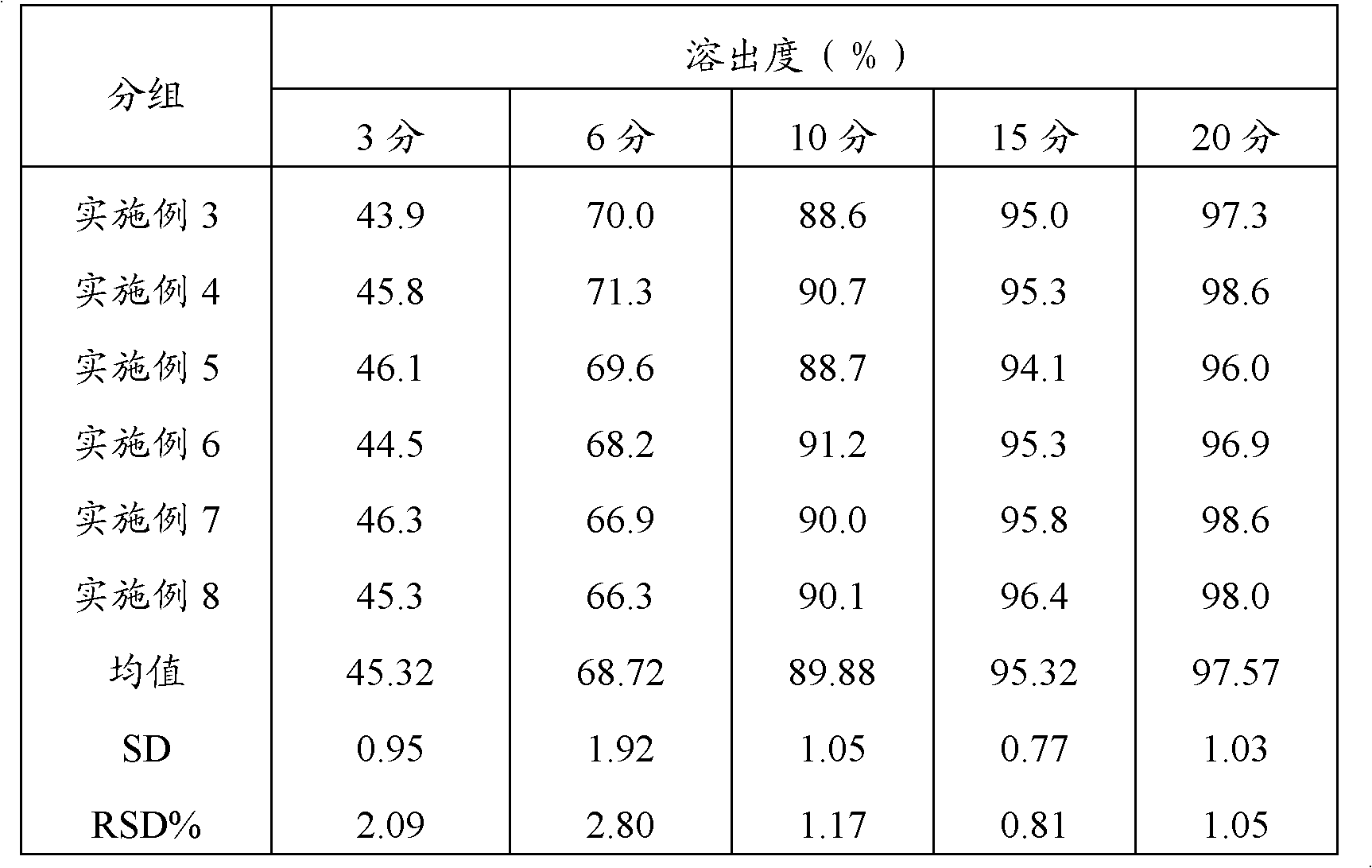
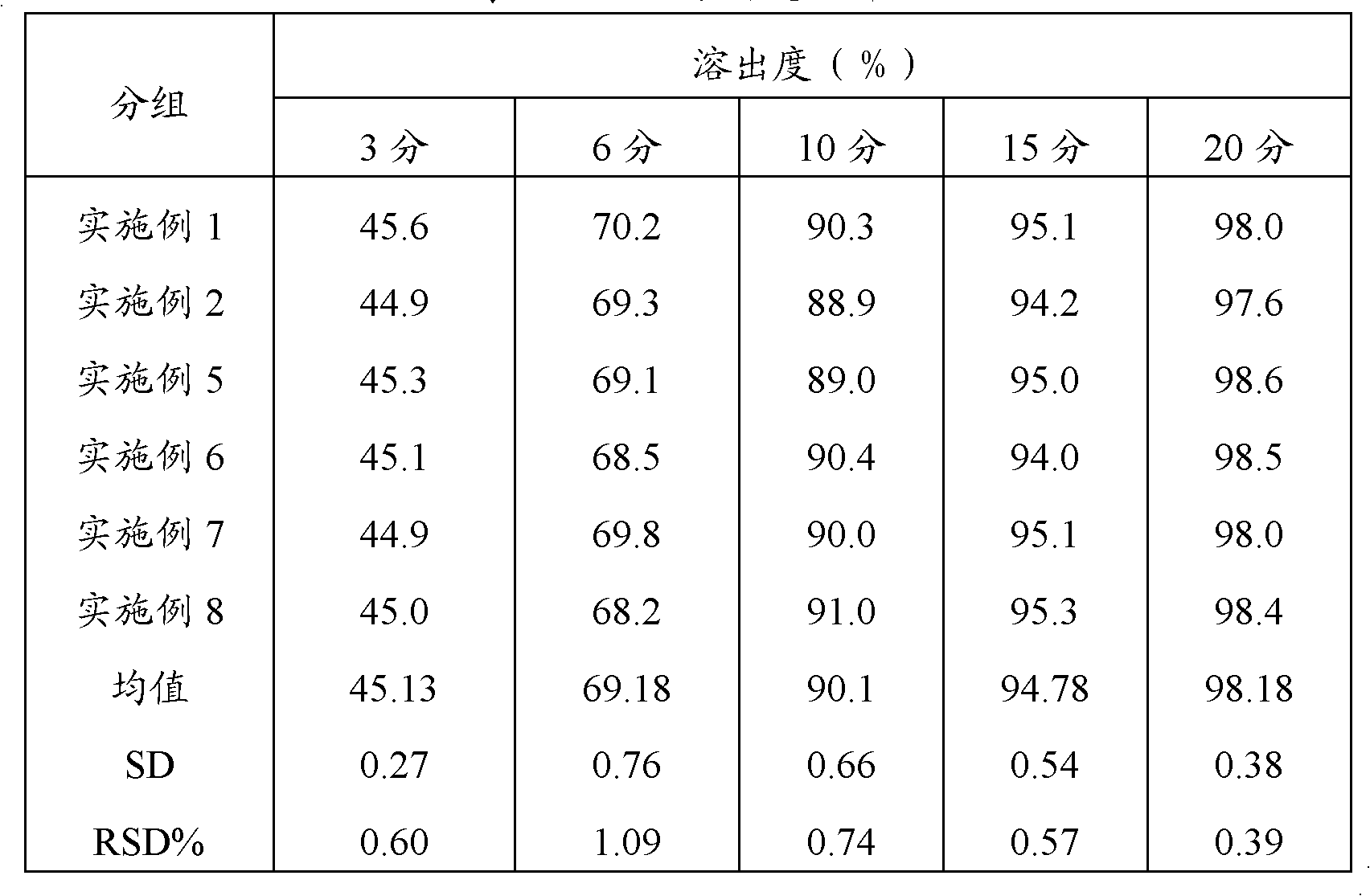
Embodiment 1
[0040] Take an appropriate amount of acetaminophen, carry out 450-mesh ultrafine grinding, weigh 20g, and set aside; weigh 230g of acetaminophen after crushing through a 100-mesh sieve, 100g of amantadine hydrochloride, 15g of caffeine, and sucrose Powder 20g, and prescription amount of artificial bezoar and chlorpheniramine maleate are added to the high-efficiency wet mixing granulator, granulated with 12% starch slurry, boiled and dried, granulated, and 5g of magnesium stearate is added , mixed uniformly, compressed into tablets, printed, packaged, inspected, and finally obtained, and 1000 pieces of compound acetaminophen tablets were prepared.
Embodiment 2
[0042] Take an appropriate amount of acetaminophen, carry out 300-mesh ultrafine grinding, weigh 100g, and set aside; weigh 150g of acetaminophen, 100g of amantadine hydrochloride, 15g of caffeine, and sucrose after crushing through a 100-mesh sieve according to the prescription amount. Powder 20g, and prescription amount of artificial bezoar and chlorpheniramine maleate are added to the high-efficiency wet mixing granulator, granulated with 12% starch slurry, boiled and dried, granulated, and 5g of magnesium stearate is added , mixed uniformly, compressed into tablets, printed, packaged, inspected, and finally obtained, and 1000 pieces of compound acetaminophen tablets were prepared.
Embodiment 3
[0044] Take an appropriate amount of acetaminophen, carry out 350-mesh ultrafine grinding, weigh 70g, and set aside; weigh 180g of acetaminophen after crushing through a 100-mesh sieve, 100g of amantadine hydrochloride, 15g of caffeine, and sucrose Powder 20g, and prescription amount of artificial bezoar and chlorpheniramine maleate are added to the high-efficiency wet mixing granulator, granulated with 12% starch slurry, boiled and dried, granulated, and 5g of magnesium stearate is added , mixed uniformly, compressed into tablets, printed, packaged, inspected, and finally obtained, and 1000 pieces of compound acetaminophen tablets were prepared.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com