Composition
a technology of compositions and compositions, applied in the field of pharmaceutical compositions, can solve the problems of inconvenient long-term treatment of sub-chronic or chronic pain, and achieve the effect of prolonging the treatment time and improving the effect of pain
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
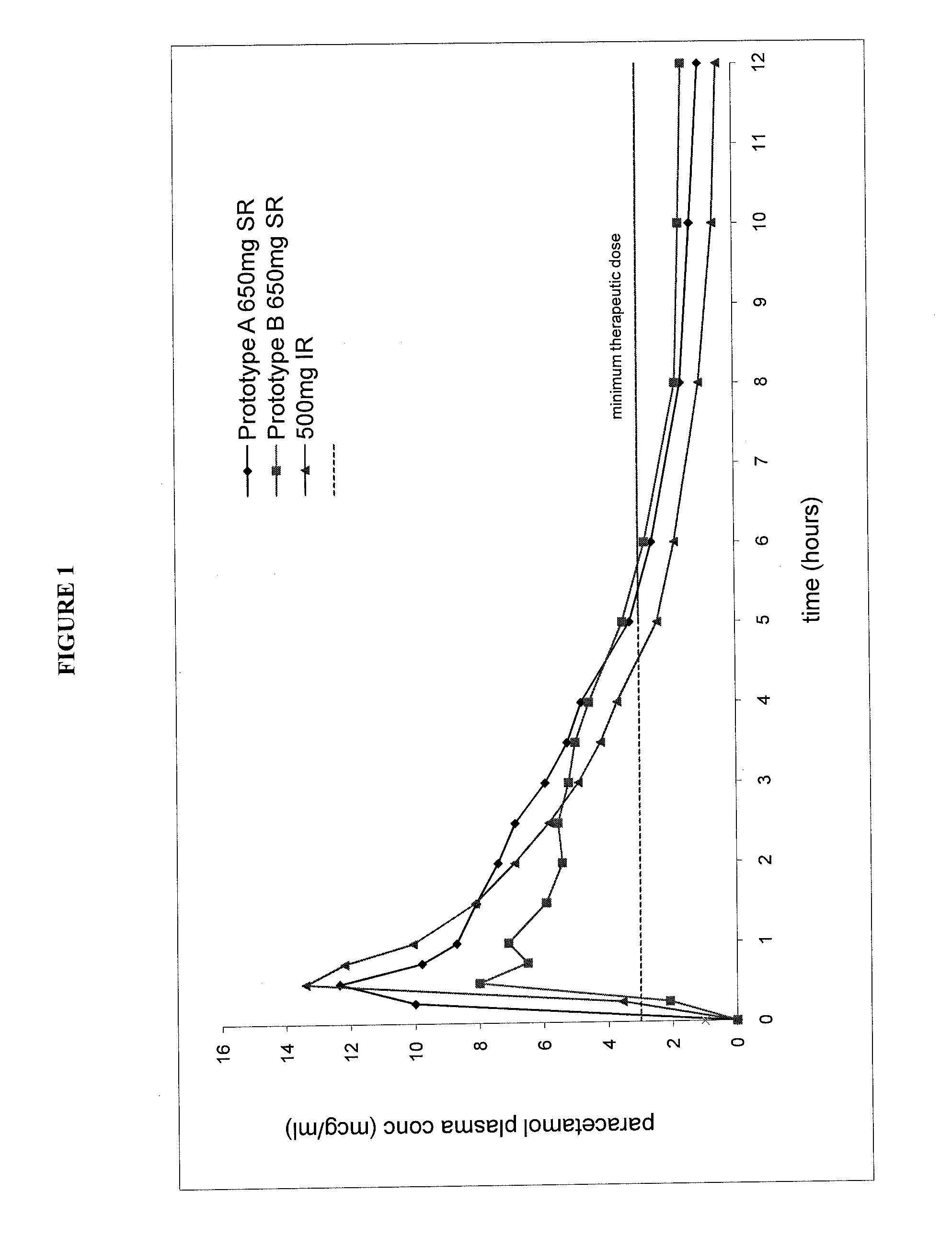
[0051]This Example compares the properties of a commercially available immediate release 500 mg paracetamol tablet with two prototype sustained release bilayer tablets (Formulations A and B) which both have an in vitro dissolution profile outside the scope of the present invention.
[0052]These prototype tablets containing a total of about 650 mg of paracetamol were prepared from the following ingredients:
Tablet Formulation ATablet Formulation BIngredientmg / tablet% w / wmg / tablet% w / wSustained Release LayerParacetamol264.0834.75403.3952.10High viscosity HPMC18.962.4928.963.74Pregelatinised Starch21.052.7732.154.15Polyvinylpyrrolidone5.880.778.981.16Low viscosity HPMC5.090.677.771.00Magnesium Stearate0.950.121.450.19Immediate Release LayerDirectly compressible436.0057.36283.536.62paracetamol granulationDC90#(Paracetamol content in(389.80)(51.28)(260.00)(33.58)DC90)Film and Wax Coating8.051.068.051.04Total760.05100.000774.25100.00% w / w SR:IR APAP41.1:59.960.5:39.5# DC90 is a commercially ...
example 2
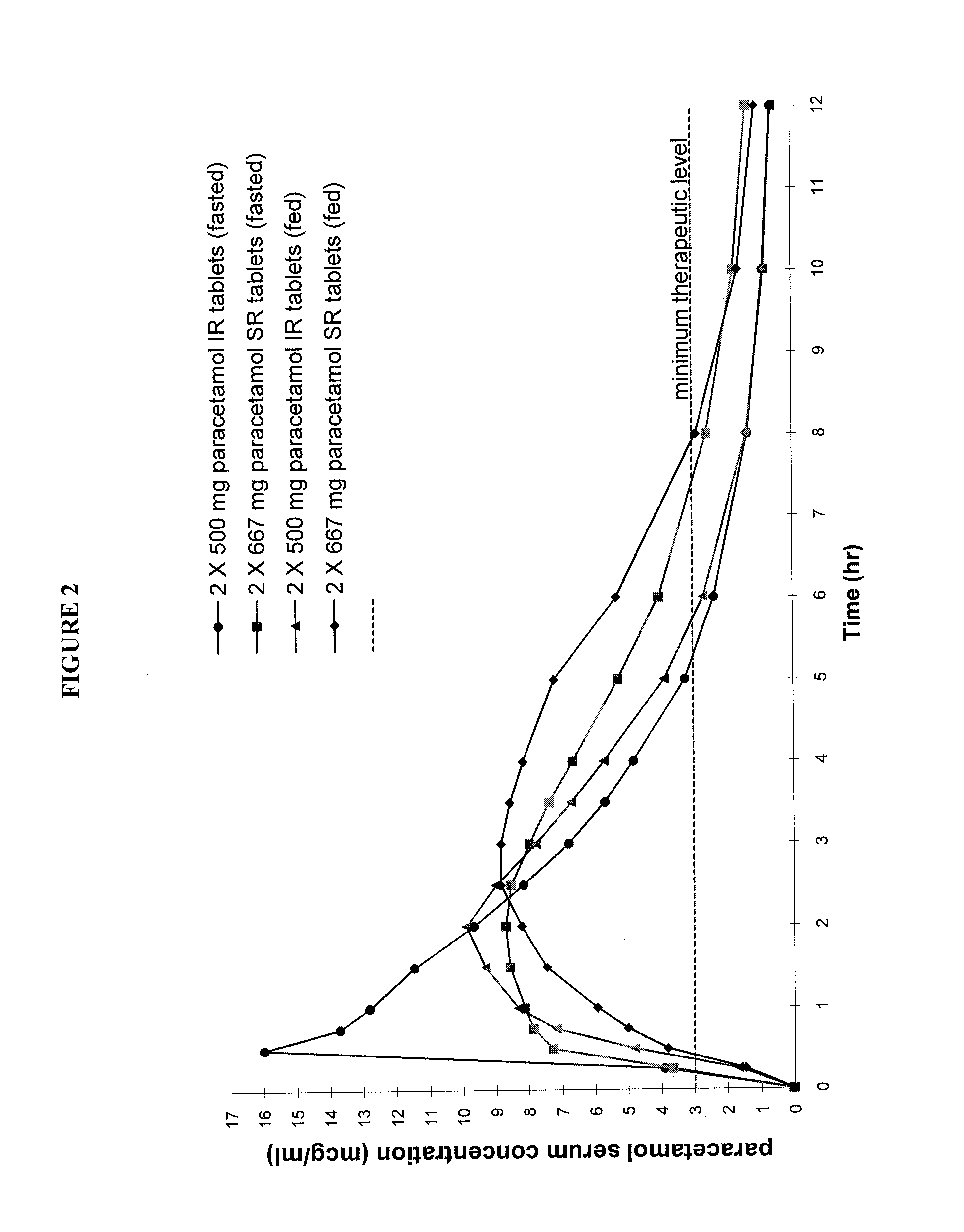
[0057]This Example compares the properties of a commercially available immediate release 500 mg paracetamol tablet with a sustained release bilayer tablet (Formulation C) having an in vitro dissolution profile falling within the scope of the present invention.
[0058]This advantageous bilayer tablet containing a total of 666.6 mg of paracetamol was prepared from the following ingredients:
Tablet Formulation CIngredientmg / tablet% w / wSustained Release LayerParacetamol473.5764.39High viscosity HPMC15.432.10Pregelatinised Starch5.140.70Polyvinylpyrrolidone10.281.40Low viscosity HPMC8.231.12Magnesium Stearate1.540.21Immediate Release LayerDirectly compressible214.9229.22paracetamol granulationDC90(Paracetamol content in(193.43)(26.30)DC90)Film and Wax Coating6.3050.86Total735.42100.00% w / w SR:IR APAP71:29
[0059]The release profile of test formulation C was characterised using the USP type III apparatus (reciprocating basket) as hereinbefore described and was found to have the following disso...
example 3
[0063]This Example compares the properties of a commercially available immediate release 500 mg paracetamol tablet with another sustained release bilayer tablet (Formulation D) having an in vitro dissolution profile falling within the scope of the present invention.
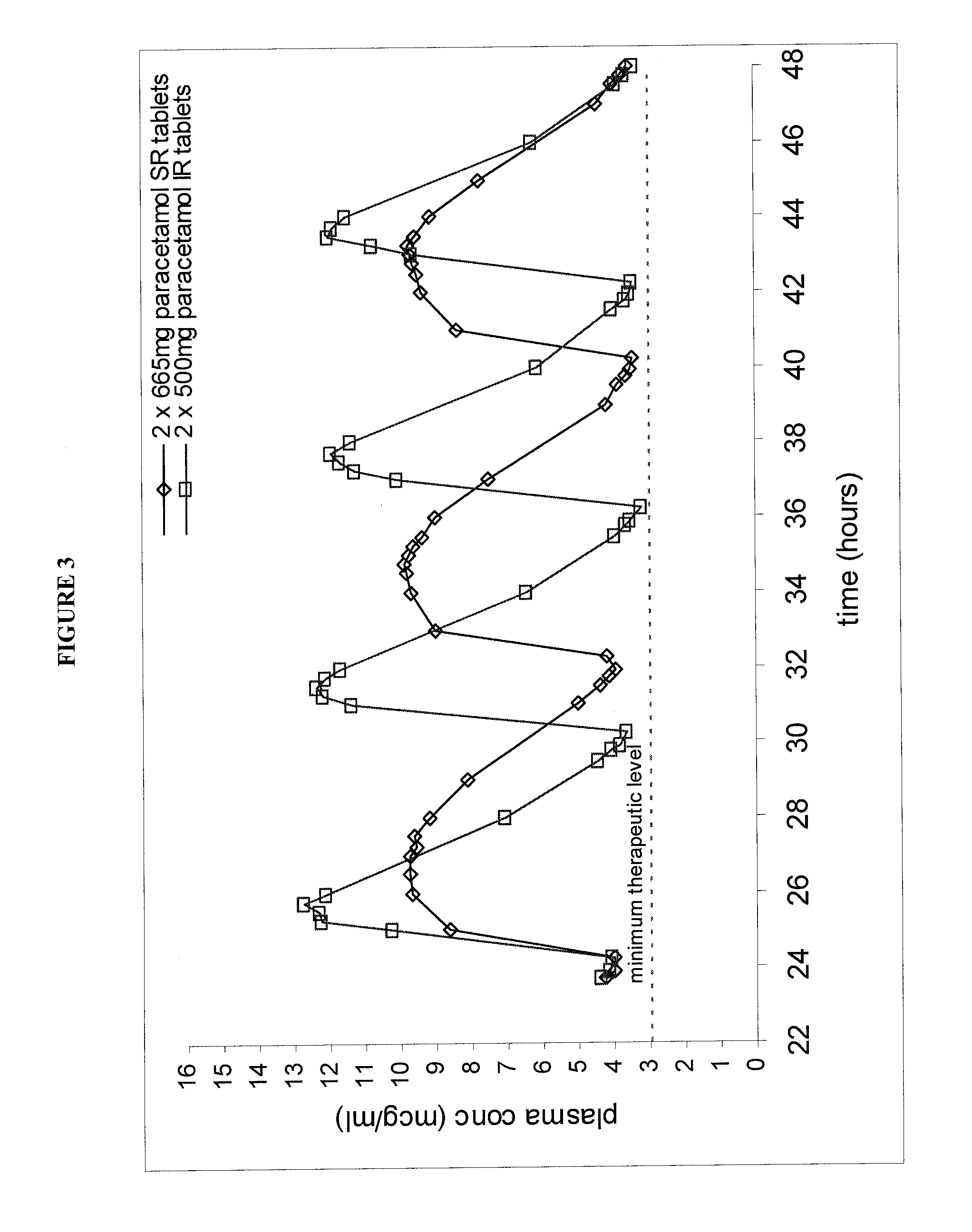
[0064]The bilayer tablet of Formulation D was essentially similar to Formulation C but contained a total of 665 mg of paracetamol and had a slightly different ratio of sustained release to immediate release paracetamol (% w / w SR:IR APAP was 69:31).
[0065]The release profile of test formulation D was characterised using the USP type III apparatus (reciprocating basket) as hereinbefore described and was found to have the following dissolution rate as detailed in table 3.
TABLE 3Dissolution Profile for Formulation DTimeIn-vitro release Results(minutes)(% paracetamol released)1540.8%6065.0%12090.2%180101.8%
[0066]Formulation D was assessed in a further biostudy which involved 27 subjects. The study was an open multiple dose cros...
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight | aaaaa | aaaaa |
| time | aaaaa | aaaaa |
| time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com