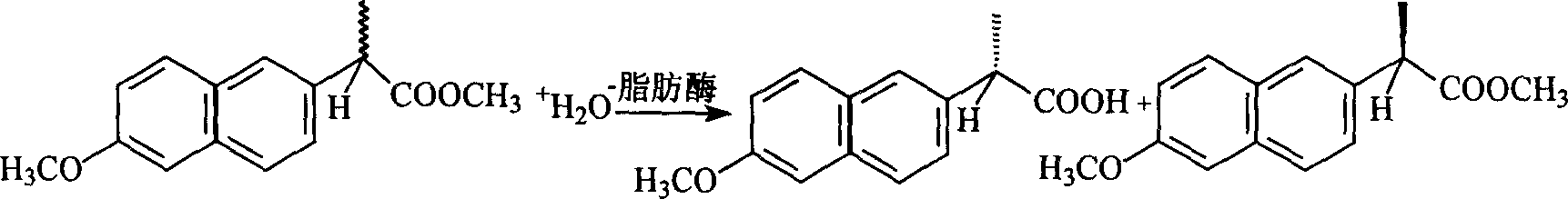
Method for biological catalytic preparing naprosyn
A technology of biocatalysis and naproxen, applied in the field of naproxen, can solve the problems of flammability, safety, low reaction rate, environmental pollution, etc., and achieve the effect of high stereoselectivity and high activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Ionic liquid [bmim] PF 6 , and water to form a two-phase system, added to a 100 ml Erlenmeyer flask, lipase L-1754, 125 mg, and 75 mg racemic naproxen methyl ester were added to the system, and in another Erlenmeyer flask Water-isooctane constitutes a two-phase system, and the addition of the same components is compared with the water-ionic liquid two-phase system.
[0019] At 32 degrees centigrade, 170 rev / min reaction, take a sample every other day, after the remaining R-naproxen methyl ester that exists in ionic liquid phase extracts with n-hexane, adopt HP1090 type high performance liquid chromatography (HP company) chiral column (OD-H), column length 250 * 4.6 millimeters, eluent is n-hexane: Virahol is 9: 1 (volume ratio), flow velocity 0.5 milliliters / min, under room temperature condition, wavelength 254nm, measure naproxen A The corresponding excess value of the ester (ee s ); the product S-naproxen present in the aqueous phase is vacuum-dried to remove water ...
Embodiment 2
[0032] Other is the same as embodiment 1, changes the ratio of water-ionic liquid two-phase, ionic liquid: water (volume ratio) A=3: 1; B=2: 1; C=2: 2; D=2: 3; E= 2:4; at 30 degrees Celsius, 170 rpm, after 5 days of reaction, take samples for measurement. The results are shown in Table 2:
[0033] The influence of table 2 water quantity on enzyme activity
[0034] reaction system ee s (%) ee p (%) E conversion rate
[0035] (C%)
[0036] A 18.44 97.97 116.8 15.84
[0037] B 27.39 98.10 136.3 21.83
[0038] C 28.6 3 98.30 154.3 22.56
[0039] D 22.03 97.71 107.1 18.40
[0040] E 21.82 97.98 121.3 18.21
[0041] The comparison found that there was an optimal ratio of ionic liquid to water in the water-ionic liquid two-phase system, and the activity and stereoselectivity of lipase reached the maximum at this ratio.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com