Method for preparing (S)-naproxen by enzyme resolution of racemic naproxen ester
A naproxen ester and naproxen technology are applied in the field of enzyme separation of racemic naproxen ester preparation-naproxen, and can solve the problem of poor dispersibility of naproxen ester, slowing down the enzymatic reaction speed, naproxen Problems such as poor solubility of raw esters, to achieve the effects of low production cost, accelerated reaction speed, high chemical purity and optical purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Example 1 Preparation of carboxylesterase BsE-NP01
[0030] Design primers:
[0031] Primer 1: 5'-TTCCATATGTCAAACCATTCATCTAGTATTCCCG-3';
[0032] Primer 2: 5'-GGATCCTTACCGTGAAATGCCTGTTTCTGCATTG-3'.
[0033] Using the extracted Bacillus subtilis CGMCC 2548 genomic DNA as a template, PCR amplification was carried out with the above-mentioned primers, and the obtained PCR product was connected to the plasmid pMD 18-T (purchased from Takara Company), transformed into Escherichia coli DH 5α, and screened. positive clone. Nucleotide sequencing was performed on the positive cloned plasmid, and the nucleotide sequence of the carboxylesterase BsE-NP01 gene of the inserted recombinant fragment was shown in SEQ ID NO.1 in the sequence table. The DNA sequence has been registered in GENBANK. The number is GQ868652. Restriction endonucleases NdeI and BamHI (purchased from Takara Company) were used to digest and ligate the recombinant plasmid obtained above and plasmid pET-11a (pu...
Embodiment 2
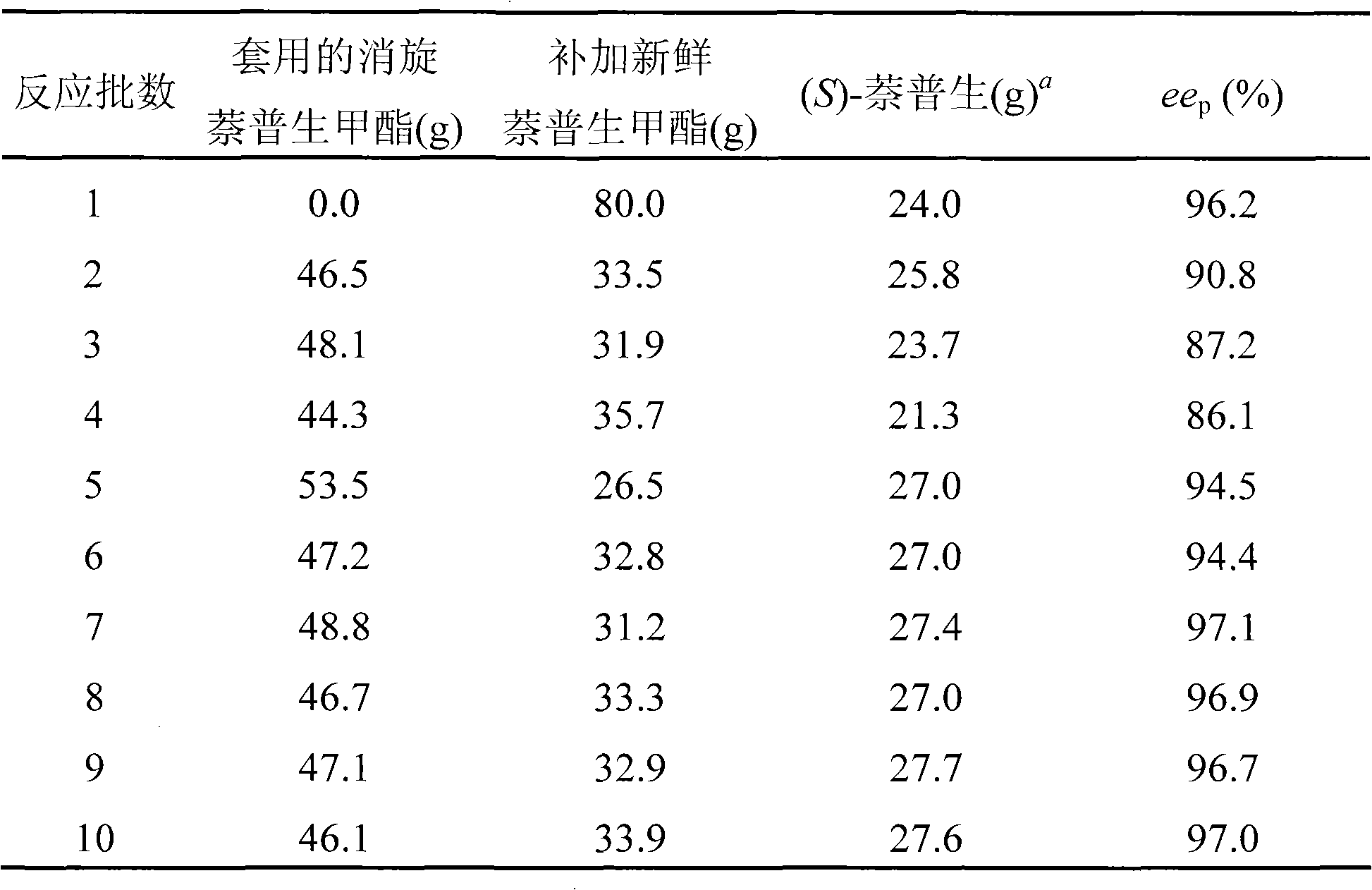
[0037] Mix 80g of racemic naproxen methyl ester with 500ml of tap water, place it in a high-speed smashing homogenizer JJ-2 (Jintan Experimental Instrument Branch of Shanghai Pudong Physical Optical Instrument Factory), and homogenize it at a high speed (10,000rpm, 20min) mashed. The suspension slurry of the gained racemic naproxen methyl ester was added and placed in a 2-L glass reactor, 600U of the crude carboxylesterase obtained in Example 1 was added, tap water was added to make up to a total volume of 2.0L, and the temperature was kept at 28 ℃ reaction, the stirring speed is 300rpm, the pH of the reaction solution is kept constant at 7.5 by automatic flow of concentrated ammonia water, and the reaction is terminated after 5 hours, and the conversion rate is about 36% at this time.
[0038] After the reaction was terminated, the reaction mixture was suction filtered, the filter cake was (R)-naproxen methyl ester that did not participate in the reaction, and the filtrate co...
Embodiment 3
[0040] The (R)-naproxen methyl ester filter cake collected in Example 2 was dried to about 50 g, 100 ml of methanol and 0.6 g of sodium methoxide were added, heated to reflux, and the reflux reaction was continued for 2 h. After cooling, suction filtration was performed to obtain racemization. Naproxen methyl ester 46.5g. The obtained racemic naproxen methyl ester can be directly mixed with fresh substrate and used for the next batch of enzymatic hydrolysis after homogenization.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com