Non-steroidal anti-inflammatory drug formulations for topical applications to the skin
a non-steroidal anti-inflammatory and skin technology, applied in the direction of biocide, drug composition, aerosol delivery, etc., can solve the problems of loss of activities, undesirable effects, and often failing to meet the stated objectives of conventional administration routes, so as to improve performance, improve performance, and improve performan
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0091] This example compares the percutaneous absorption through porcine skin, of ibuprofen from aqueous alcoholic gels containing 5 wt. % ibuprofen and either 5%, 10% or 15% of 2-n-nonyl-1,3-dioxolane, using an ethanol / water carrier at a 70:30 mixing ratio. The formulations include NaOH to adjust the pH to 7.4, but do not include a glycol. Hydroxypropyl cellulose (2 wt. %) is used as the gelling agent. The test compositions are applied to provide about 30 milligrams (mg) of the composition per square centimeter (cm2) of porcine skin.
[0092] The tests are run in standard static cells with phosphate buffered saline (PBS) as the receptor fluid (surface area 0.635 cm2, temperature 32° C.). The following Table 1 shows the total amount of ibuprofen applied to the skin for each formulation. The differences result from the slightly different thicknesses at which the test formulations are applied.
[0093] Each test was run for 24 hours under non-occluded conditions with the finite dose of th...
example 2
[0096] This example shows the effect of incorporating propylene glycol in the aqueous alcoholic gel formulation containing 5% ibuprofen and 10% 2-n-nonyl-1,3-dioxolane using an ethanol:water vehicle at a 70:30 weight mixing ratio. The compositions used in these tests are shown in Table 3 (NaOH is added to adjust the pH to 7.4):
TABLE 3propyleneibuprofenenhancerglycolEthanolWaterTotal(%)(%)(%)(%)(%)(%)A510059.525.5100B51055624100C5101052.522.5100D510154921100E5102045.519.5100
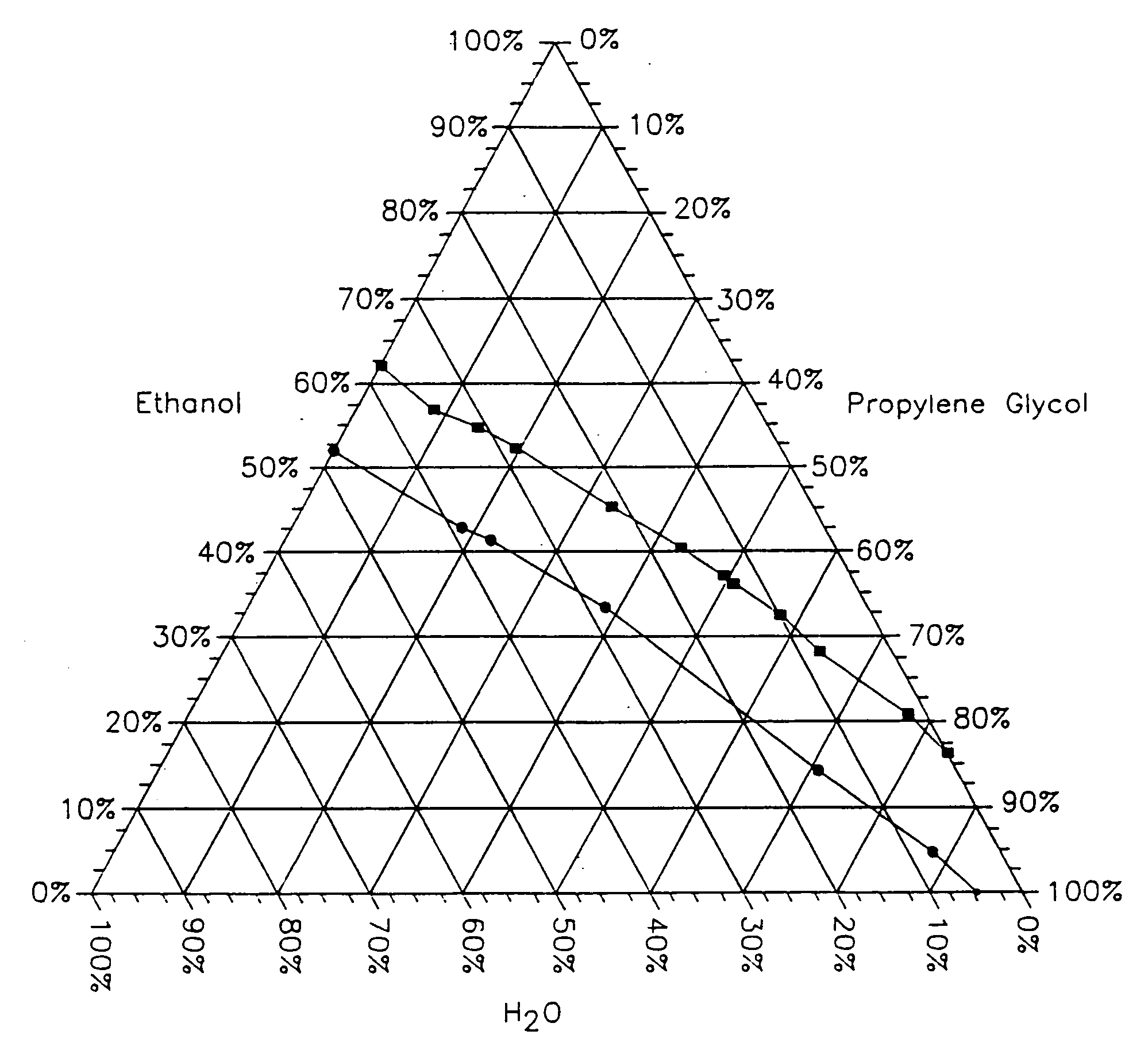
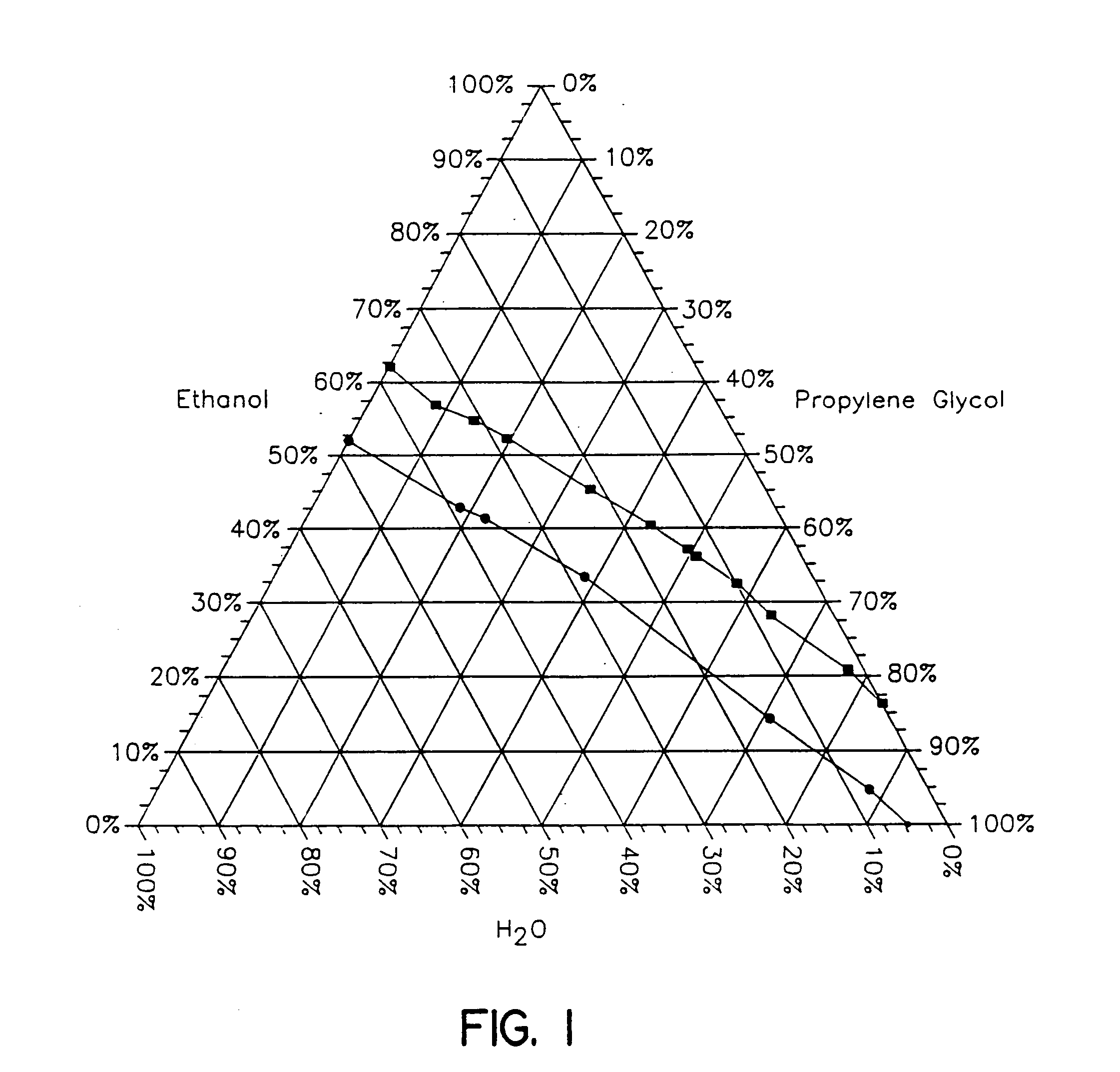
[0097] The test was run using the same conditions as described in Example 1. The flux was measured at 2, 4 and 6 hours. The results are shown graphically in FIG. 3. From this figure it is seen that the flux at 2 hours decreases nearly linearly as the propylene glycol (PG) content increases from 0% to 5% to 10% to 15% to 20%. At four hours after the composition is applied to the test skin sample the fluxes for each concentration of PG has increased but more so for the compositions containing the higher amounts of...
example 3
[0099] This example is similar to Example 1 but compares a topical aqueous alcoholic gel formulation with ibuprofen according to the present invention with a similar gel but without the enhancer and with four other commercially available topical ibuprofen formulations. Also, human skin was used rather than porcine skin. The composition according to the present invention and the comparison were as follows:
InventionComparisonIngredientAmount (wt. %)Amount (wt. %)Ibuprofen552-n-nonyl-1,3-100dioxolaneEthanol5965Propylene glycol1719Water79Hydroxypropyl22celluloseSodium Hydroxideq.s. to pH 7q.s. to pH 7
[0100] The commercially available products were: Gelufene® (ibuprofen 5%, isopropyl alcohol, hydroxyethylcellulose, sodium hydroxide, benzyl alcohol and purified water), Dolgit® cream (ibuprofen 5%, medium chain triglycerides, mixture of glycerol monostearate and polyoxyethylene stearates, polyoxyethylene fatty acid esters, xanthan gum, lavender oil, neroli oil, water, propylene glycol, p...
PUM
| Property | Measurement | Unit |
|---|---|---|
| surface area | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com