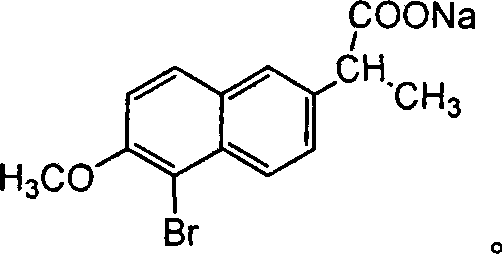
Industrial synthesis technique for DL-naproxen
A synthesis process and technology of the process, applied in the field of industrial synthesis process, can solve problems such as low yield, unstable bromide, easy decomposition, etc., and achieve the effect of ensuring operability, saving processing steps and raw materials, and ensuring continuity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
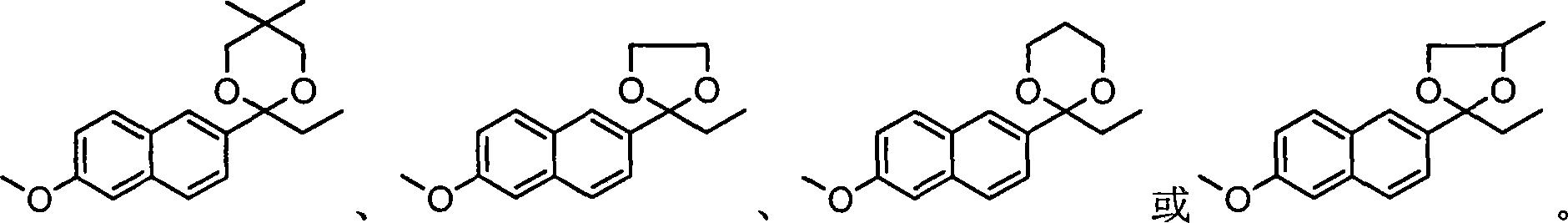
[0097] Put 30g (0.14mol) of propionylnaphthyl ether, 3g of PTS, 20g (0.19mol) of neopentyl glycol into a 500ml three-necked bottle, add 300ml of toluene, install a reflux device with a water separator, and heat up and reflux for 5 to 7 hours , be down to room temperature, add 1% sodium bicarbonate solution 100ml, stir, a large amount of crystals are separated out, filter, filter cake is washed once with 100ml water and 100ml toluene respectively, dry, obtain about 42g (0.14mol) of product I, yield Close to 100%, mp: 131-132°C; content (HPLC) 99.5%.
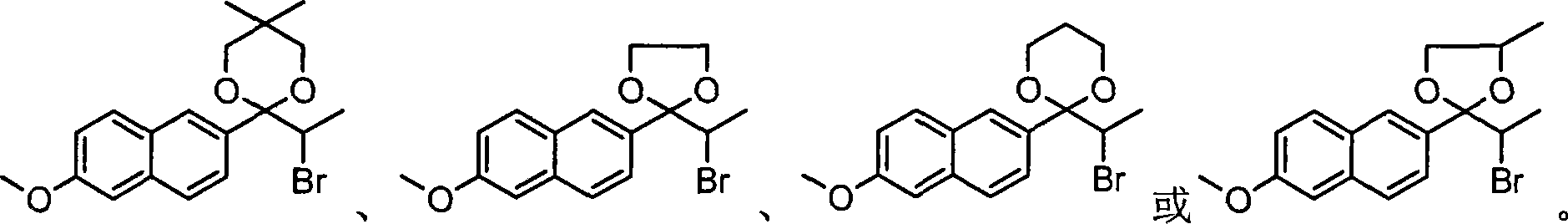
[0098] Put 30g (0.1mol) of ketal I into a dried 500ml three-necked bottle, add 300ml of dichloroethane, cool down to 10°C, put 38.4g of PTT into the three-necked bottle, and stir for 2 to 5 hours, naturally Warm up to room temperature, add 100ml of water to wash, separate the water layer, then wash with 100ml of 2% aqueous sodium bicarbonate solution, separate the water layer, finally wash with 100ml of water until neutral, dry wi...
Embodiment 2
[0107] Put 30g (0.14mol) of propionylnaphthyl ether, 3g of PTS, 16g (0.21mol) of 1,2-propanediol into a 500ml three-necked bottle, add 300ml of cyclohexane, install a reflux device with a water separator, and heat up and reflux for 10 ~14 hours, be down to room temperature, add 1% sodium bicarbonate solution 100ml, stir, have a large amount of crystals to separate out, filter, filter cake is washed once with 100ml water and 100ml hexanaphthene respectively, dry, obtain about 42g of product I (0.14 mol), the yield is close to 100%, and the content (HPLC) is 99.5%.
[0108] Put 30g (0.1mol) of ketal into a dried 500ml three-necked flask, add 300ml of dichloromethane, cool down to 10°C, put 38.4g of PTT into the three-necked flask, stir for 2 to 5 hours, and naturally heat up to At room temperature, add 100ml of water to wash, separate the water layer, then wash with 100ml of 2% sodium bicarbonate aqueous solution, separate the water layer, finally wash with 100ml of water until ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com