Non-steroidal antiinflammatory drug formulations for topical application to the skin
a non-steroidal anti-inflammatory and skin technology, applied in the direction of drug compositions, aerosol delivery, bandages, etc., can solve the problems of loss of activities, undesirable effects, and often failing to meet the stated objectives of conventional administration routes, so as to improve performance, improve performance, and improve performan
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
third embodiment
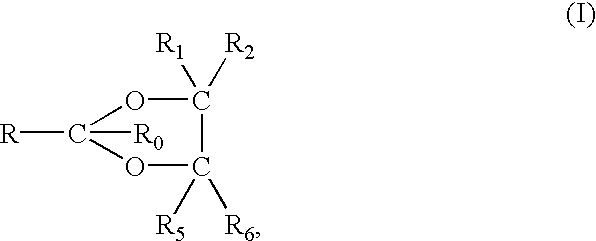
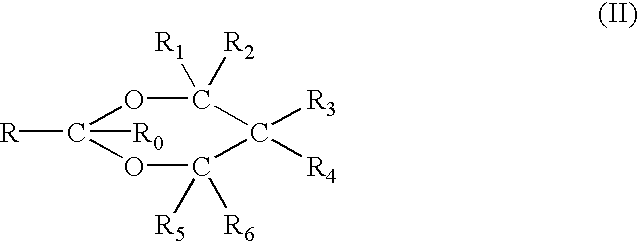
[0085] The vehicle for any of the forms of the compositions of the invention will include glycol, e.g., propylene glycol, butylene glycol, hexylene glycol, etc. (except in the case of the third embodiment described above), lower alcohol, e.g., ethanol, isopropanol, and, usually, water. A thickening or gelling agent is also usually and preferably included to facilitate application of the formulation to the skin. In addition, of course, the skin penetration enhancing dioxolane, dioxane or acetal is included in the formulations in an amount effective to enhance the penetration of the active NSAID ingredient through the skin, including the stratum corneum.
[0086] Accordingly, the vehicle or carrier system for the NSAID and enhancer components is preferably an aqueous or non-aqueous alcoholic carrier containing sufficient alcohol, especially ethanol and / or isopropanol and, often, glycol, e.g., propylene glycol, to solubilize the NSAID and be miscible with the enhancer. Generally, however,...
example 1
[0091] This example compares the percutaneous absorption through porcine skin, of ibuprofen from aqueous alcoholic gels containing 5 wt. % ibuprofen and either 5%, 10% or 15% of 2-n-nonyl-1,3-dioxolane, using an ethanol / water carrier at a 70:30 mixing ratio. The formulations include NaOH to adjust the pH to 7.4, but do not include a glycol. Hydroxypropyl cellulose (2 wt. %) is used as the gelling agent. The test compositions are applied to provide about 30 milligrams (mg) of the composition per square centimeter (cm.sup.2) of porcine skin.
[0092] The tests are run in standard static cells with phosphate buffered saline (PBS) as the receptor fluid (surface area 0.635 cm.sup.2, temperature 32.degree. C.). The following Table 1 shows the total amount of ibuprofen applied to the skin for each formulation. The differences result from the slightly different thicknesses at which the test formulations are applied. Each test was run for 24 hours under non-occluded conditions with the finite d...
example 2
[0095] This example shows the effect of incorporating propylene glycol in the aqueous alcoholic gel formulation containing 5% ibuprofen and 10% 2-n-nonyl-1,3-dioxolane using an ethanol:water vehicle at a 70:30 weight mixing ratio. The compositions used in these tests are shown in Table 3 (NaOH is added to adjust the pH to 7.4):
3 TABLE 3 propylene ibuprofen enhancer glycol Ethanol Water Total (%) (%) (%) (%) (%) (%) A 5 10 0 59.5 25.5 100 B 5 10 5 56 24 100 C 5 10 10 52.5 22.5 100 D 5 10 15 49 21 100 E 5 10 20 45.5 19.5 100
[0096] The test was run using the same conditions as described in Example 1. The flux was measured at 2, 4 and 6 hours. The results are shown graphically in FIG. 3. From this figure it is seen that the flux at 2 hours decreases nearly linearly as the propylene glycol (PG) content increases from 0% to 5% to 10% to 15% to 20%. At four hours after the composition is applied to the test skin sample the fluxes for each concentration of PG has increased but more so for t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| surface area | aaaaa | aaaaa |
| wt. % | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com