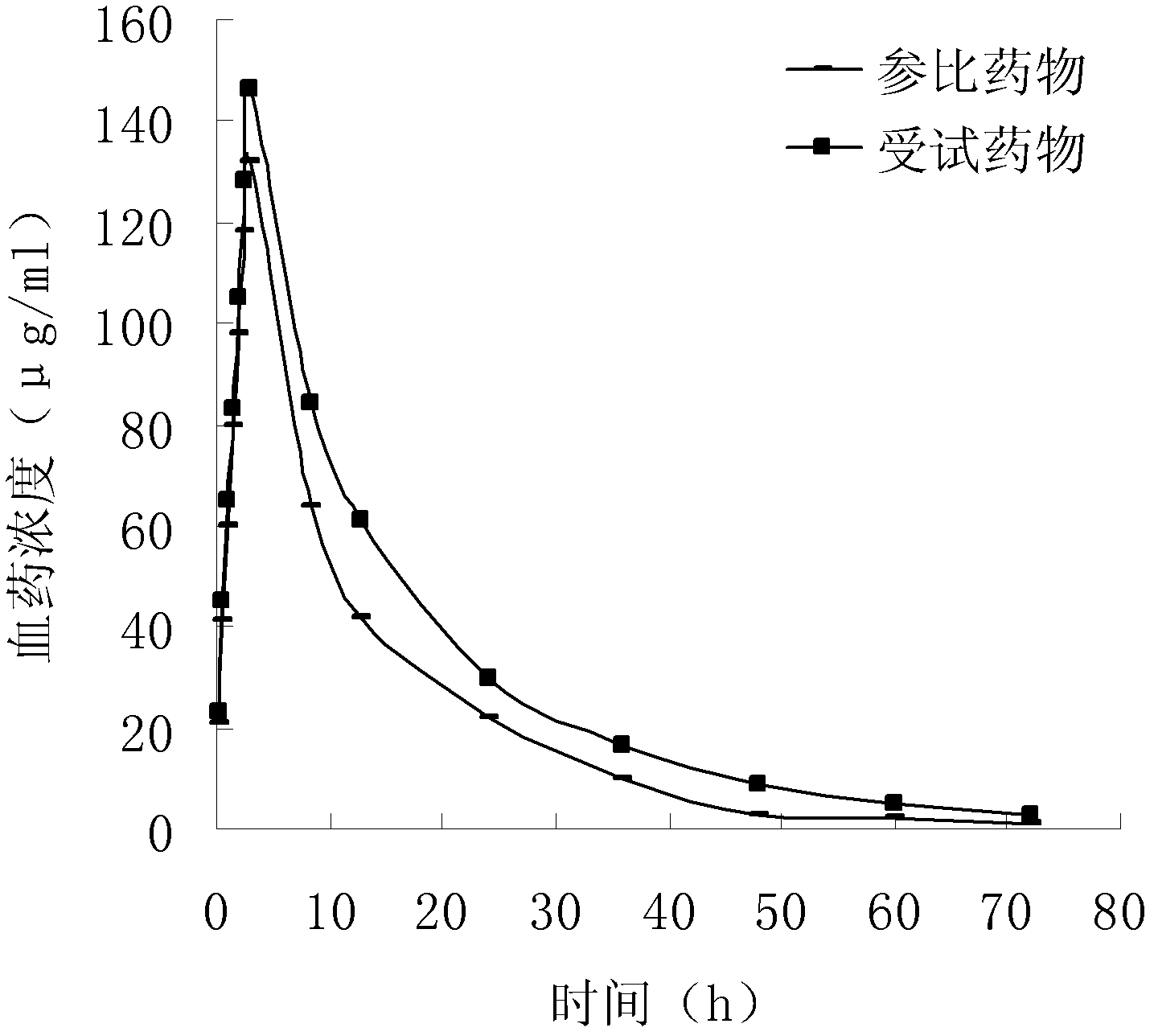
Naproxen hydrate crystal, preparation method thereof and pharmaceutical composition containing the crystal and sumatriptan
A hydrate and naproxen technology, applied in the field of medicine, can solve problems such as low solubility, large gastrointestinal irritation, affecting drug dissolution effect and bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0075] [Example 1] Preparation of naproxen hydrate crystals
[0076] (1) Cool the mixed solution of solvent ethanol 30ml and acetone 25ml to 6°C, add 10g naproxen to dissolve to obtain solution 1;
[0077] (2) In an ultrasonic field with a power of 0.5kw, add 48ml of deionized water dropwise to solution 1 until crystals precipitate; the temperature of solution 1 is controlled at 6°C during the dropping process;
[0078] (3) After dropping, close the ultrasonic field, maintain the above temperature and stir for 1.5 hours, then stand at 6°C for 3.5 hours, and filter; the filter cake is washed with 10ml purified water and 15ml ethyl acetate, and dried in vacuum to obtain naproxen Hydrate crystals.
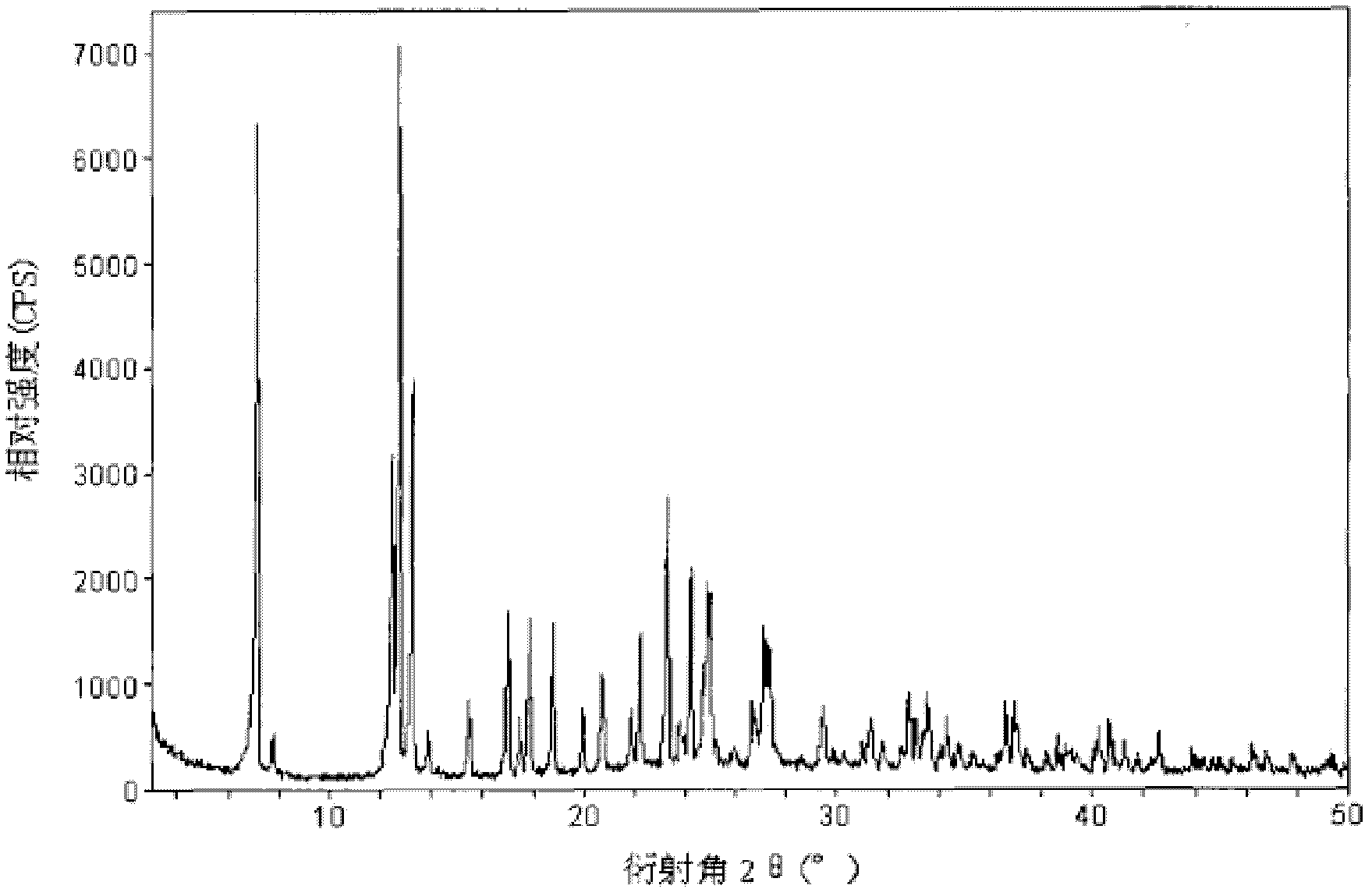
[0079] The X-ray powder diffraction spectrum obtained by Cu-Kα ray measurement of the prepared naproxen hydrate crystals (see figure 1 The characteristic peaks in 2θ are displayed at 7.3°, 12.5°, 13.0°, 13.5°, 17.0°, 17.9°, 18.8°, 22.1°, 23.4°, 24.2°, 25.0° and 27.4°.
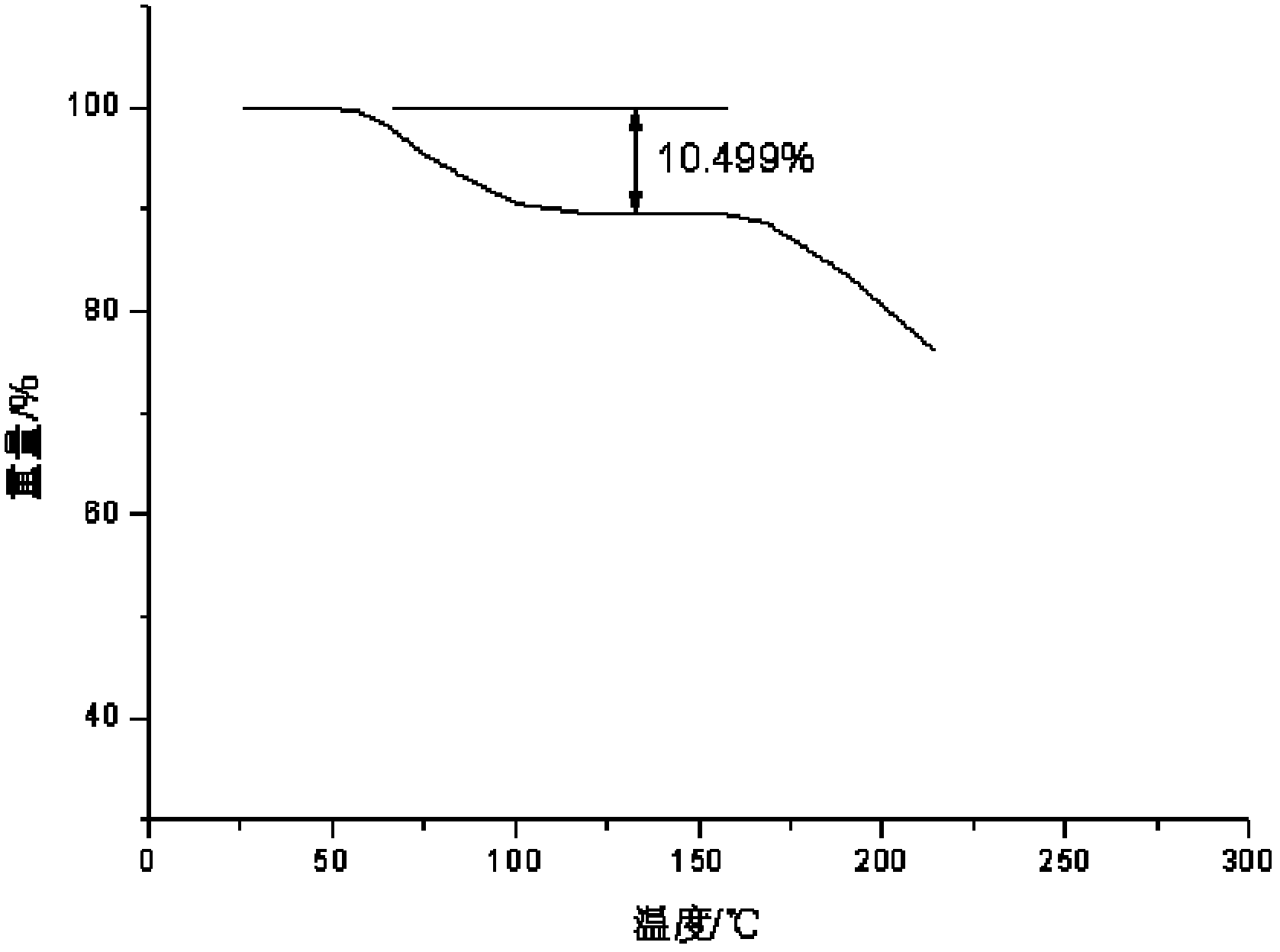
[0080] Using PE Pyris D...
preparation Embodiment 1
[0085] [Formulation Example 1] Preparation of Naproxen / Sumatriptan Tablets
[0086] The prescription is as follows:
[0087]
[0088]
[0089] Preparation:
[0090] 1) Preparation of naproxen and sumatriptan package
[0091] i) Crush the amount of sumatriptan through an 80 mesh sieve for use;
[0092] ii) Weigh the naproxen hydrate crystals of Example 1 according to the stated amount, and mix them with the above-mentioned crushed and sieved sumatriptan to obtain a mixture of naproxen and sumatriptan;
[0093] iii) Heat and melt the gelatin in the stated amount, add the above-mentioned mixture of naproxen and sumatriptan, stir evenly, and pulverize through an 80-mesh sieve after cooling to obtain the naproxen and sumatriptan package.
[0094] 2) Preparation of naproxen / sumatriptan tablets
[0095] i) The amount of pharmaceutical excipients compressible starch, microcrystalline cellulose, low-substituted hydroxypropyl cellulose, cross-linked polyvinylpyrrolidone, sodium carboxymethyl cellulo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com