Methods and compositions for liver specific delivery of therapeutic molecules using recombinant AAV vectors
a technology of aav and a composition is applied in the field of methods and compositions for liver specific delivery of therapeutic molecules using recombinant aav vectors, which can solve the problems of generalization of aav infection, limited usefulness as a vehicle to deliver large sequences, etc., and achieve the effects of preventing or limiting transcription, preventing or limiting the expression of over-produced, and preventing terminal glycosylation and translation of the molecul
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 2
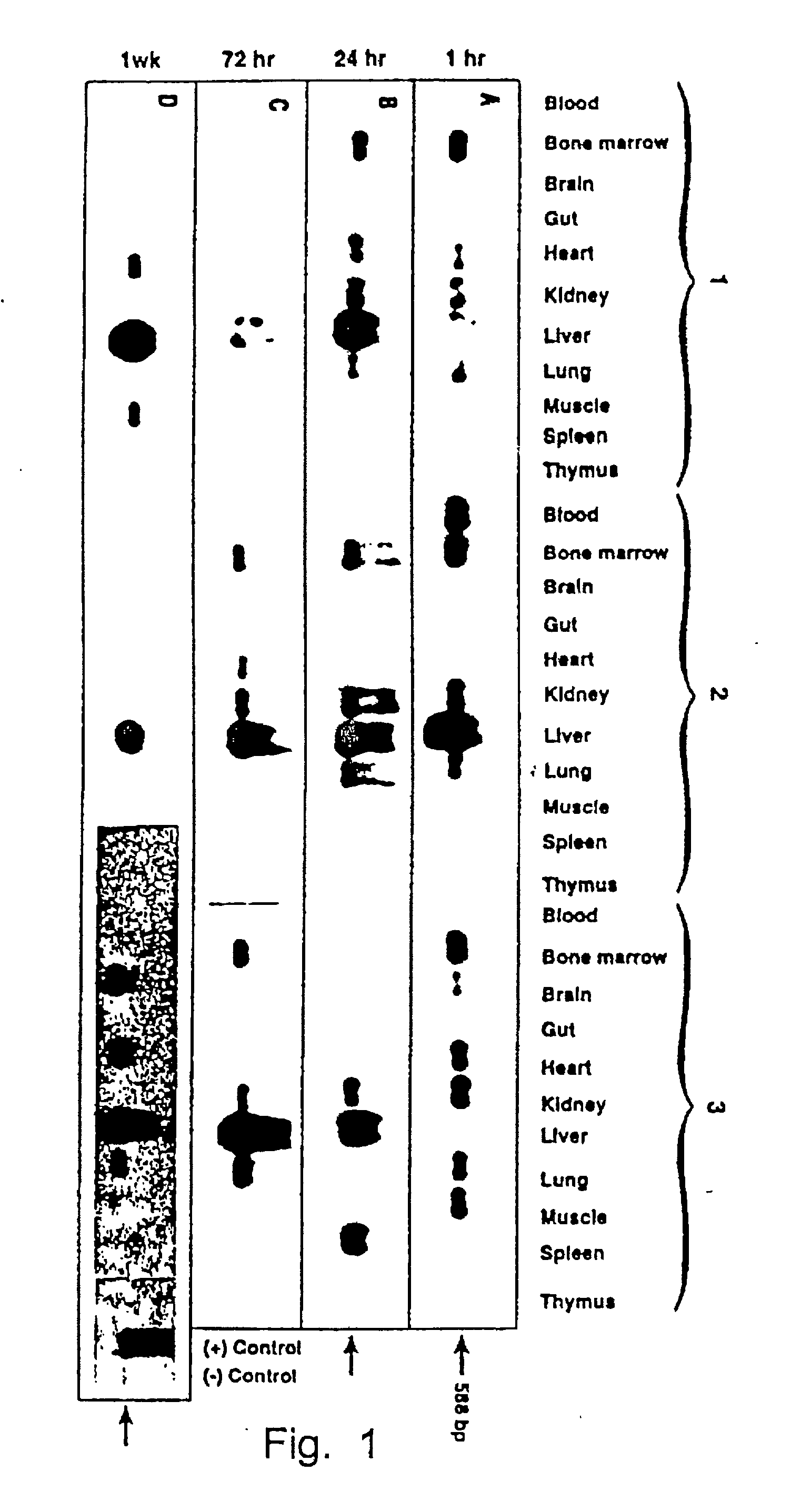
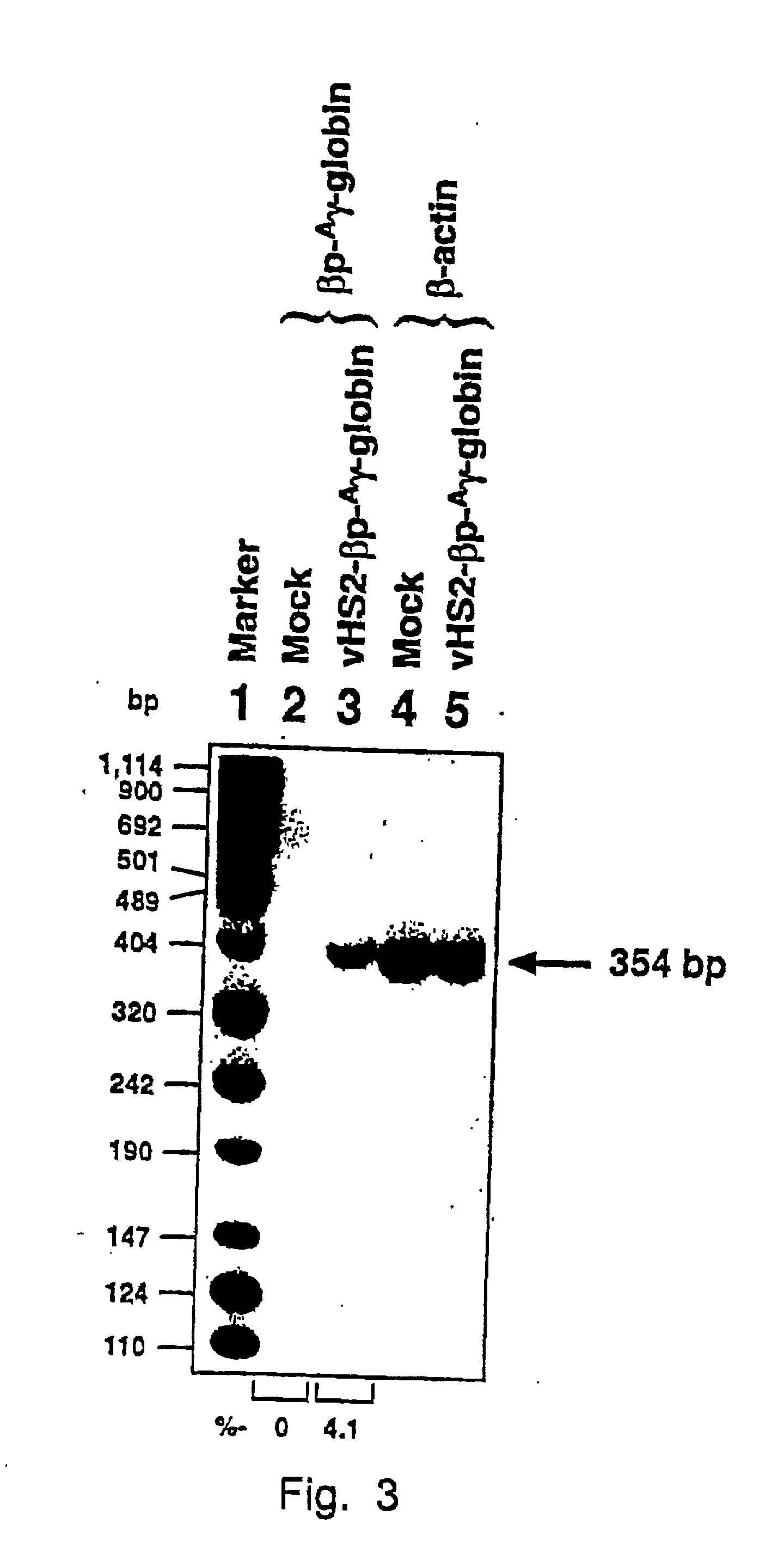
[0048] The results in Example 1 were corroborated by injecting recombinant vHS2-.beta.p-.sup.A.gamma.-globin virions under conditions identical to those in Example 1 and examining tissues from various organs seven weeks p.i. using the same techniques, but employing a .beta.-globin promoter-.sup.A.gamma.-globin gene-specific primer-pair.
[0049] Highly purified recombinant AAV vectors containing the human .beta.-globin promoter-driven human .sup.A.gamma.-globin gene containing the DNase hypersensitive-site 2 (HS-2) enhancer element (see Tuan, An erythroid specific, development stage-independent enhancer far upstream of the human ".beta.-like globin" genes, Proc. Natl. Acad. Sci. 86 (1989) 2554-2559) from the locus control region (LCR) from the human .beta.-globin gene cluster (vHS2-.sup.-A.gamma.-globin) were directly injected into C57B1 / 6 mice.
[0050] Approximately 1.times.10.sup.10 particles of the vHS2.beta.p-.sup.A.gamma.-globin r-virus were injected i.v. as described in Example 1. ...
example 3
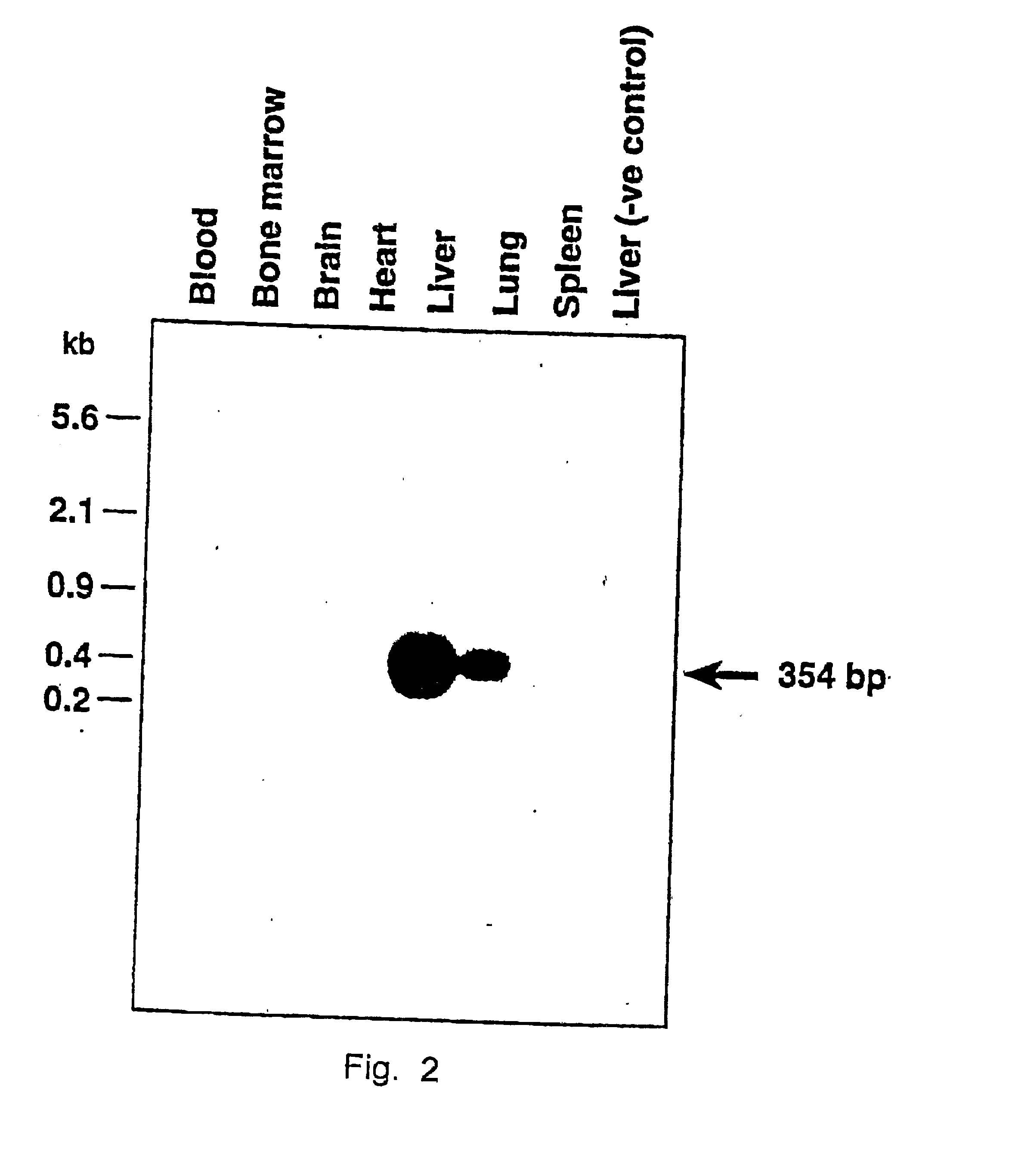
[0052] We next examined whether the lacZ gene delivered by direct injection of the r-AAV was transcriptionally active. Livers from mock-injected and vCMVp-lacZ-injected C57B1 / 6 mice were obtained one week p.i., and cryopreserved. Tissue sections were fixed and stained with 5Bromo-4-chloro-3-indolyl-.beta.-D-galactopyranoside (XGal) as described in Cheng, Separable Regulatory Elements Governing Myogenin Transcription in Mouse Embryogenesis, Science 261 (1993) 215-218 and visualized under a light microscope.
[0053] Livers were obtained one week p.i. and frozen immediately in iso-pentane at -40.degree. C. Sections of 15 .mu.m were prepared using a cryostat and fixed in a solution containing 2% formaldehyde / 0.2% para-formaldehyde in phosphate-buffered saline (PBS, 135 mM NaCl / 2.5 mM KCl / 8 mM Na.sub.2HPO.sub.4 / 0.6 mM KH.sub.2PO.sub.4 / 0.55 mM dextrose / liter) for 5 min on ice, washed twice with PBS and stained overnight at 37.degree. C. in a solution containing 5 mM K.sub.3Fe(CN).sub.6 / 5 mM...
example 4
[0054] Co-transfection of an rAAV vector containing the AAV ITRs and the nucleic acid sequence encoding a therapeutic molecule and a helper plasmid containing the necessary rep and cap functions into adenovirus-2 (Ad2) infected 293 cells was expected to eliminate homologous recombination events leading to the production of contaminating wild-type (wt) AAV during the production of recombinant vector stocks. However, contaminating "wild type-like AAV" particles have been observed in such stocks ranging from 0.1% to 10%.
[0055] To determine the mechanism of generation of contaminating wt AAV, stocks were amplified through four successive round of co-infection with Ad2 in 293 cells. Low molecular weight DNA fragments were isolated, digested with BalI restriction endonuclease and molecularly cloned into a pBlueScript plasmid vector. AAV sequence-positive clones were subjected to nucleotide sequencing using T3 and T7 primers. Nucleotide sequence analysis of 12 independent clones revealed t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| nucleic acid sequence | aaaaa | aaaaa |
| stability | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com