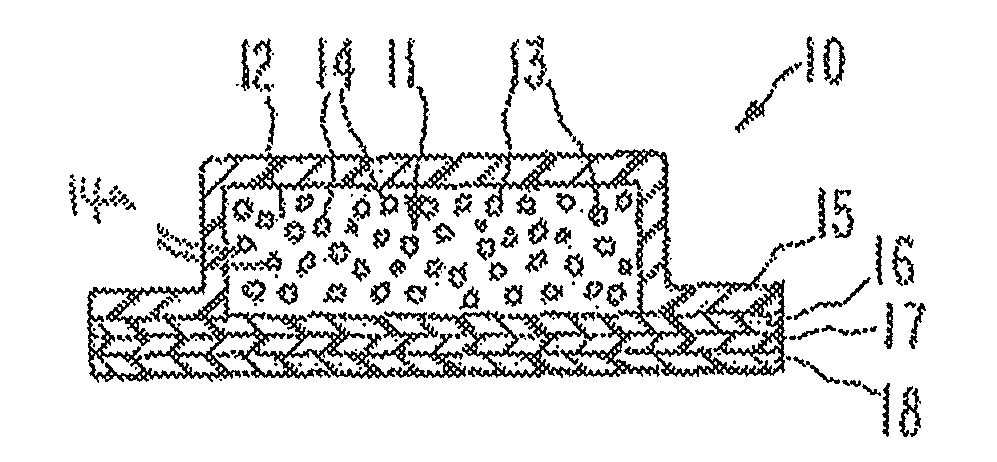
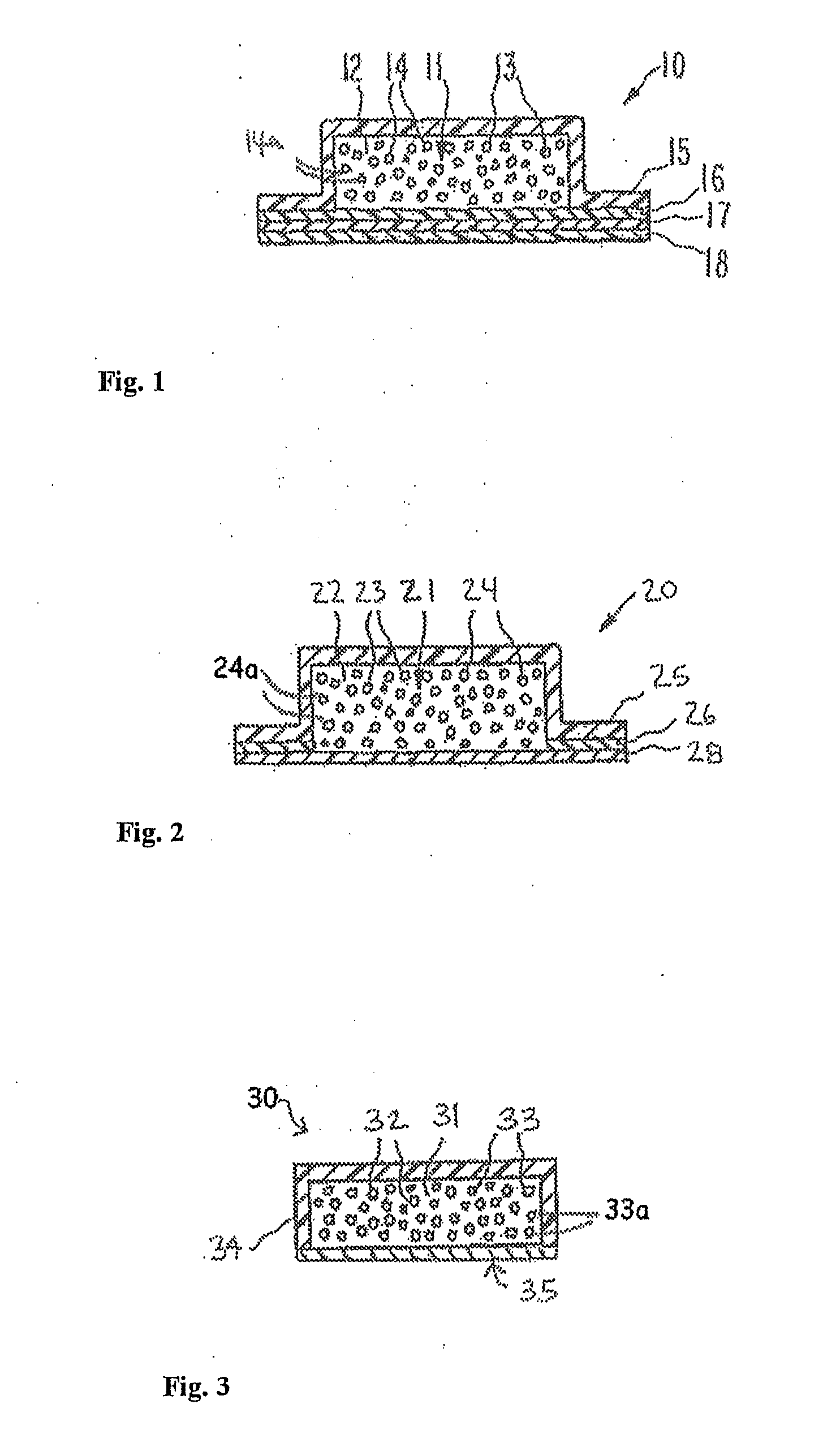
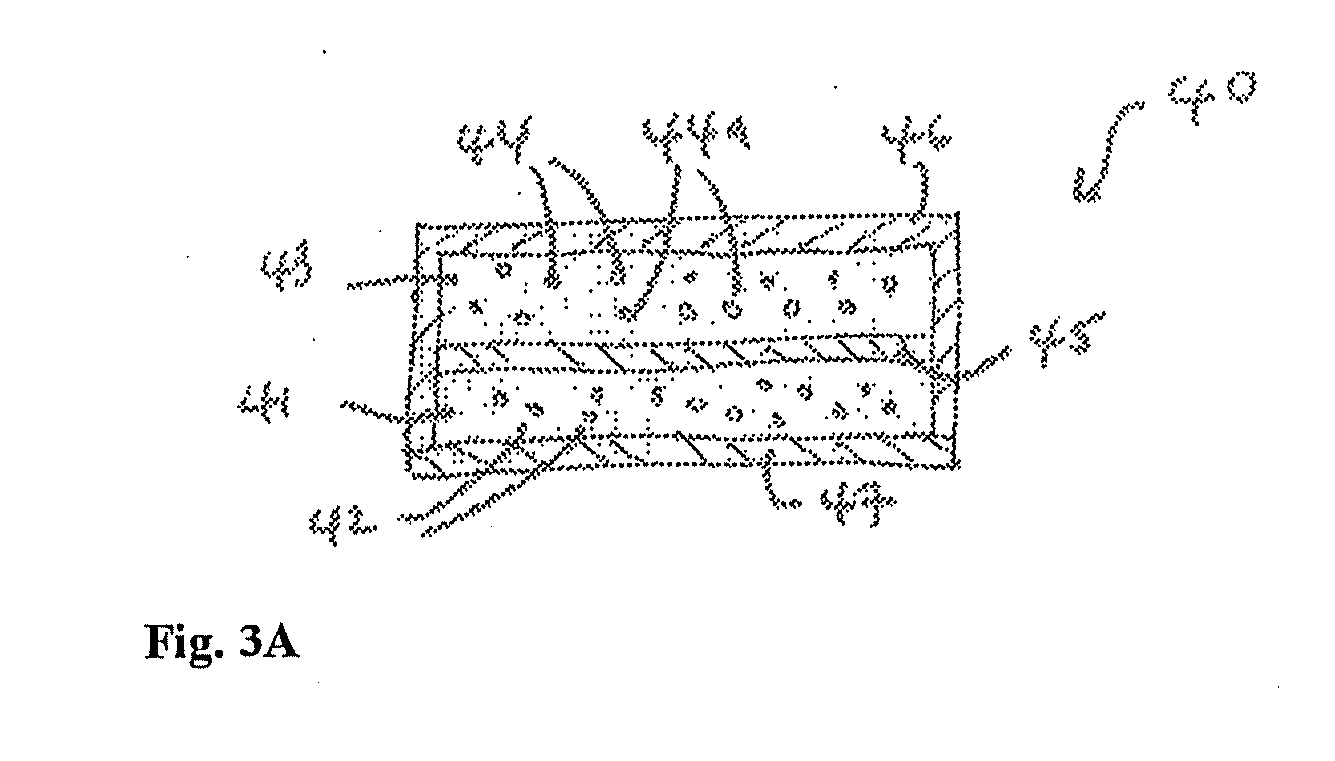
Transdermal dosage form comprising an active agent and a salt and a free-base form of an adverse agent
a technology of adverse agent and active agent, which is applied in the direction of biocide, bandages, drug compositions, etc., can solve the problems that the careful disposal of used dosage forms might not be completely effective for preventing abuse, and the amount of active agent remaining in the dosage form is susceptible to intentional or inadvertent abuse or misuse, and the effect of inhibiting the euphoric effect of the opioid
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0088] A transdermal dosage form according to FIG. 3A was prepared, with the first adhesive layer comprising 15 mg / cm2 fentanyl free base in adhesive. The second adhesive layer comprised 15 mg / cm2 nalmefene HCl in adhesive. Several 2 cm2 portions of the device were die-cut, the protective liner removed, the portions immobilized and added to testing vessels. The amount of fentanyl free base and of nalmefene HCl extractable from the device (and thus potentially available for abuse) in some cases contained within the transdermal disposal device, was determined through extraction using the following solvents: phosphate-buffered saline (“PBS”), alcohol / water mixture and ethyl ether. 15 mL aliquots of solvent were used in each extraction. The PBS solution was prepared by combining equal amounts of a 0.1M phosphate buffer (pH 6.5) and a 0.5M solution of sodium chloride. The alcohol / water mixture was prepared by mixing 75 parts absolute ethanol with 25 parts water.
[0089] The test sample vi...
example 2
[0092] A transdermal dosage form according to FIG. 3A was prepared, with the first adhesive layer comprising 15 mg / cm2 fentanyl free base in adhesive. The second adhesive layer comprised approximately equal amounts of naltrexone and nalmefene HCl in adhesive, at a total loading of 15 mg / cm2. Several 2 cm2 portions of the device were die-cut, the protective liner removed, the portions immobilized and added to testing vessels. The amount of fentanyl free base, nalmefene HCl and naltrexone extractable from the device (and thus potentially available for abuse) in some cases contained within the transdermal disposal system, was determined through extraction using the following solvents: PBS, alcohol / water mixture and diethyl ether. 15 mL aliquots of solvent were used in each extraction. The PBS solution was prepared by combining equal amounts of a 0.1M phosphate buffer (pH 6.5) and a 0.5M solution of sodium chloride. The alcohol / water mixture was prepared by mixing 75 parts absolute etha...
example 3
[0097] Table 6 shows the data obtained after 30 minutes for the patches tested in Examples 1 and 2.
TABLE 6EXTRACTION DATA AT 30 MINUTESPBSEthanol / WaterDiethyl Ether(μg)(μg)(μg)Example 1Fentanyl25640974339Nalmefene HCl15834353Fentanyl:Antagonist17.04.912.3RatioExample 2Fentanyl30645133646Nalmefene HCl357211346Naltrexone38735534Fentanyl:Antagonist4.23.11.9Ratio
The ratios recited are the amount of extracted fentanyl divided by the total amount of extracted agonist.
[0098] As shown in Table 6, extraction of an illustrative transdermal dosage form of the invention results in a mixture of an Opioid and Antagonist. The ratio of extracted Opioid to total extracted Antagonist in a patch having an Opioid, Antagonist Free Base and Antagonist Salt is lower than that of a transdermal device having only an Opioid and Angonist Salt. Because extraction of the illustrative transdermal dosage form provides a lower ratio of extracted Opioid to extracted Antagonists relative to a device that contain...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Mass | aaaaa | aaaaa |
| Mass | aaaaa | aaaaa |
| Mass | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com