In-vitro performance testing system and testing method of transcatheter bicuspid valve valved stent
A mitral valve valve and testing system technology, which is applied in the field of testing devices for cardiac valve performance testing, can solve the problem of high risk, inability to test the influence of important tissue structures around the mitral valve valve stent fixation feature, left ventricular outflow tract stenosis, etc. problem, to achieve the effect of reducing costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0047] In order to better understand the above technical solutions of the present invention, a further detailed description will be given below in conjunction with the drawings and embodiments.
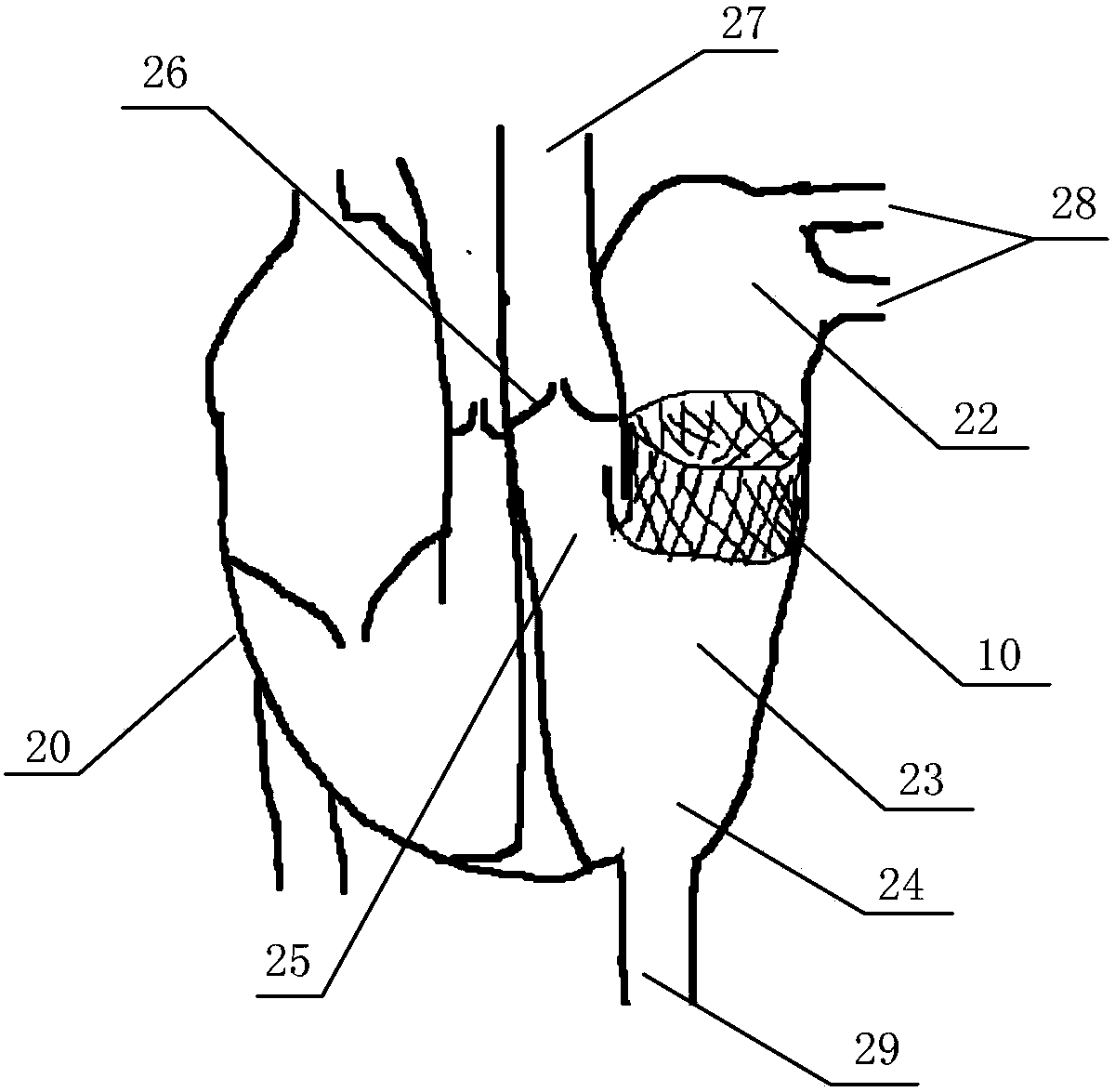
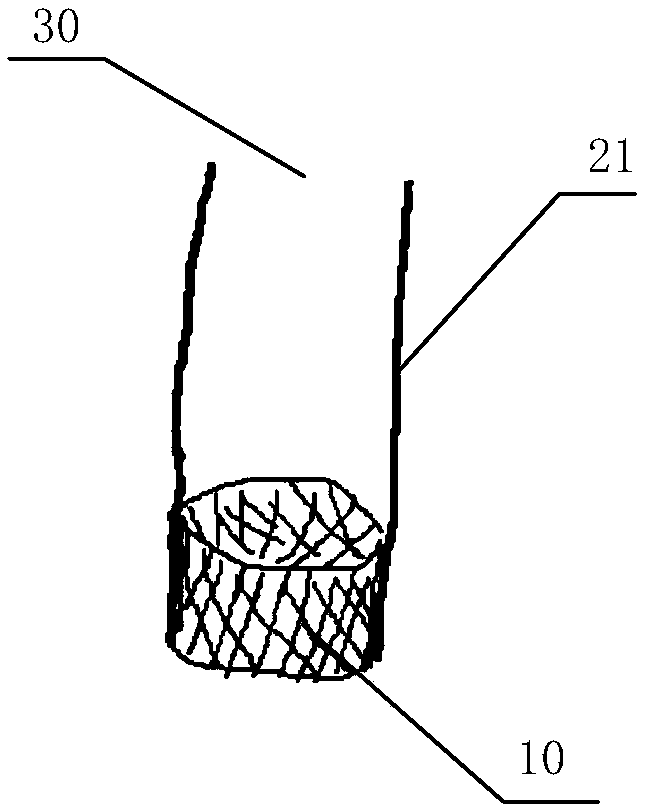
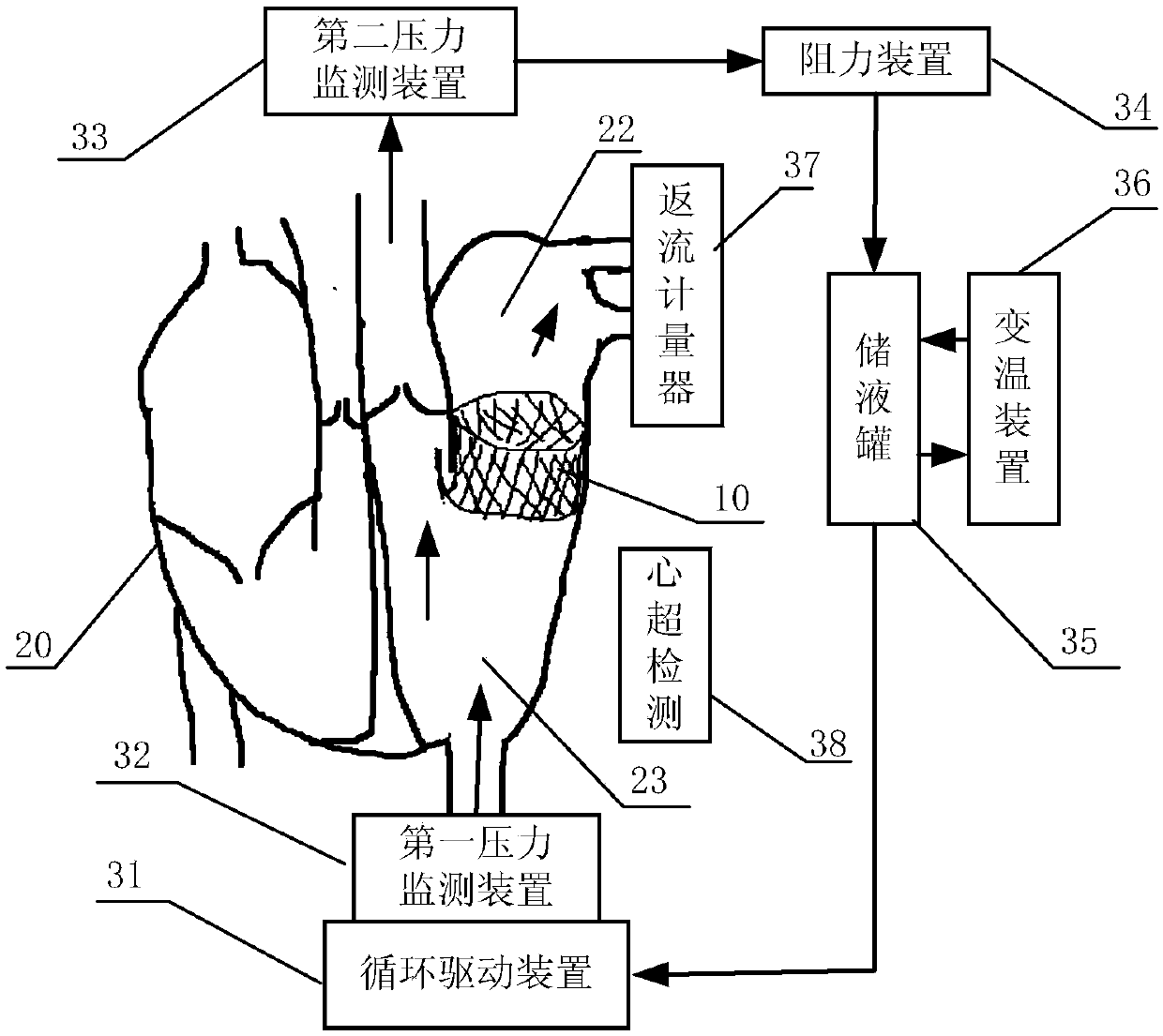
[0048] Figure 3 to Figure 5 It is an embodiment of the in vitro performance testing system of the transcatheter mitral valve stent of the present invention as shown, including the valve testing part built in the mitral valve stent 10 to be tested, and the constant flow or pulsating flow cycle provided by the valve testing part A liquid circulation device for liquid, and a monitoring device for monitoring the performance index of the valve stent;
[0049] The valve testing component includes a first testing part 20 and a second testing part 21;
[0050] The first test part 20 is a heart sample with a circulation inlet 29 at the apex of the left ventricle; the heart sample includes an isolated animal heart or a simulated heart made of artificial materials, and the circulation inlet 29...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com