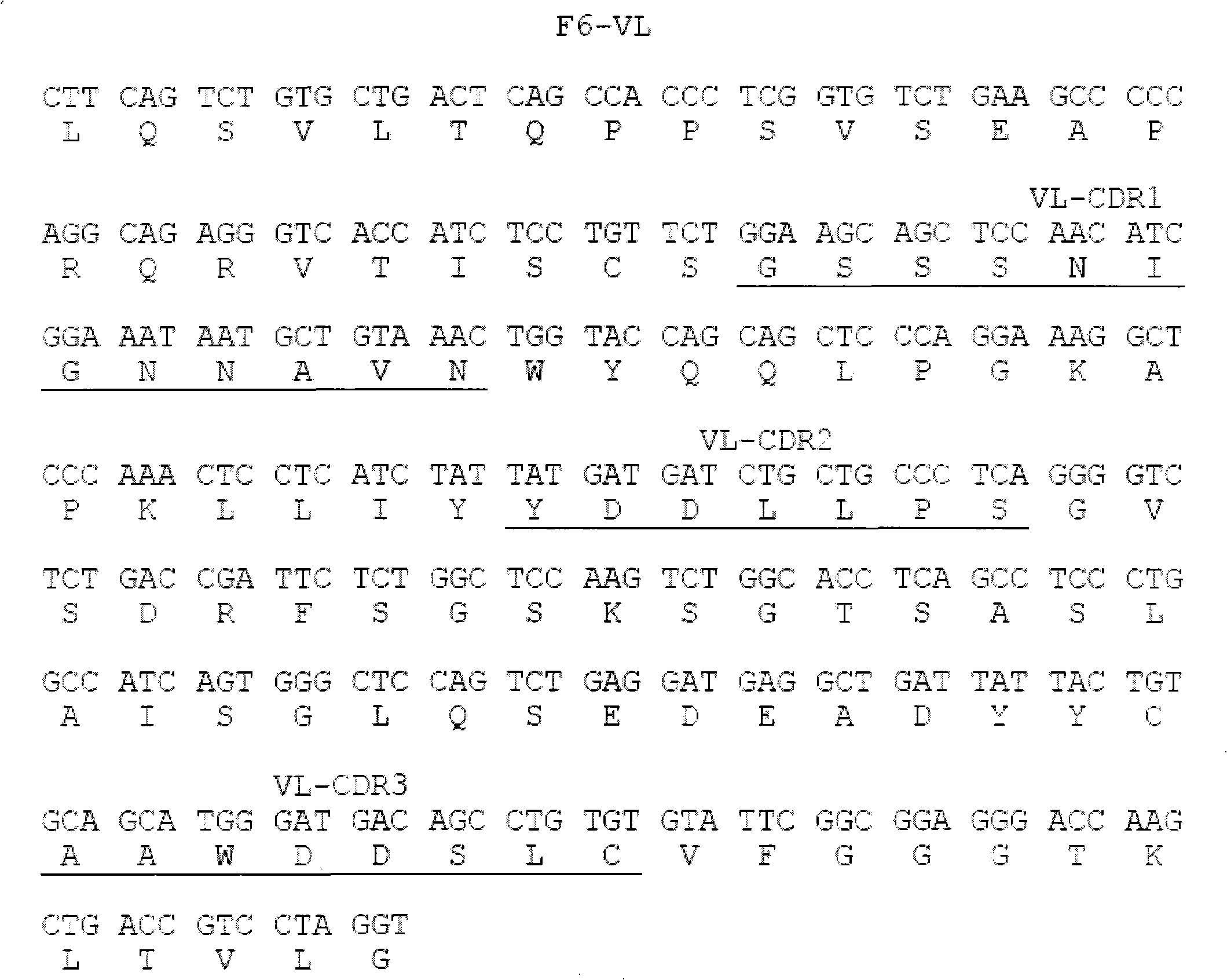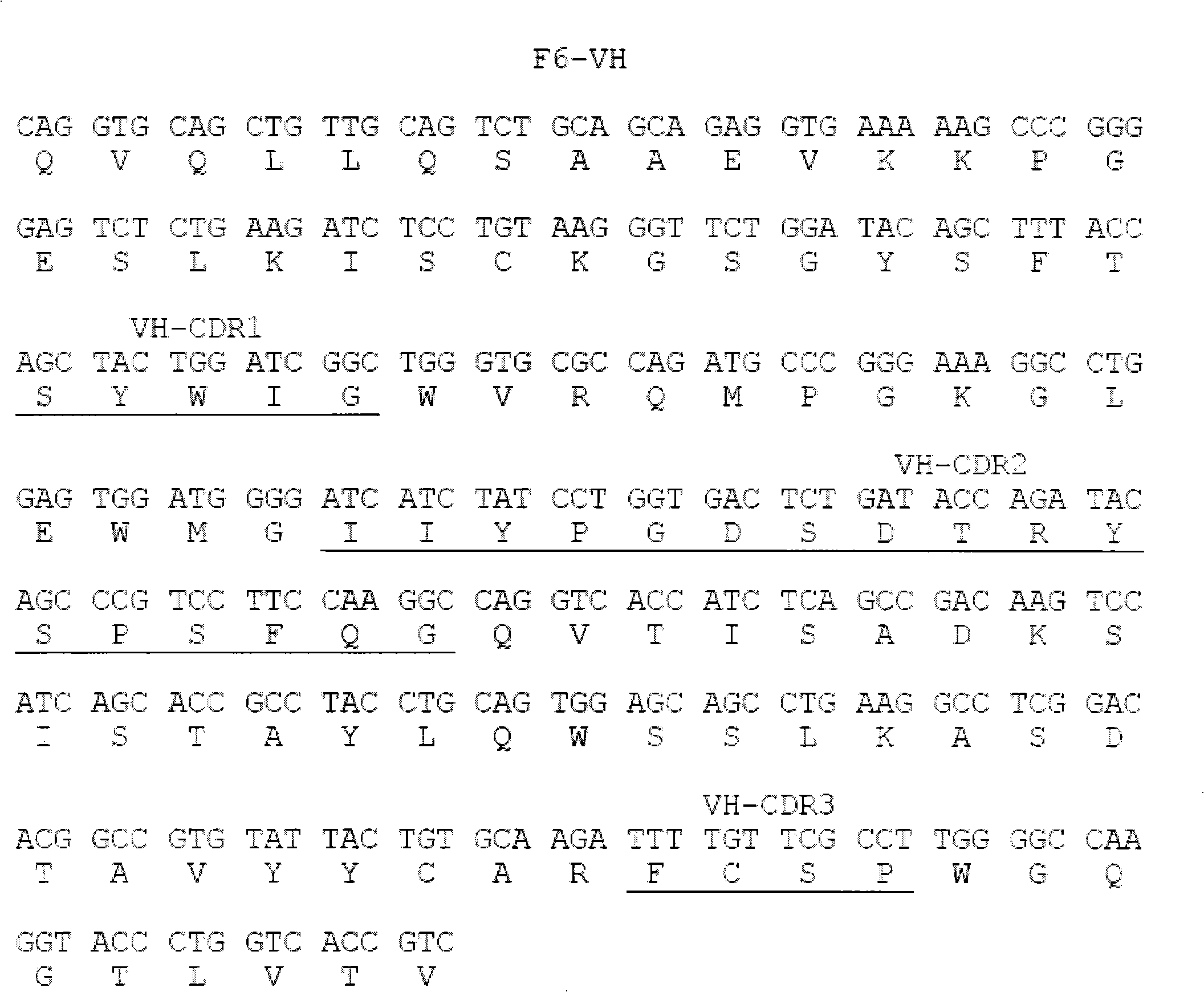Human anti-human tumor necrosis factor-alpha single chain antibody
A fully human antibody and antigen technology, applied in the field of bioengineering
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] Example 1 Screening of fully human anti-hTNF-α single-chain antibody
[0016] Coating buffer (50mM NaHCO 3 , pH9.6) to dilute hTNF-α to 100μg / ml; take 4ml and add it to an immune tube, and coat overnight at 4°C; the next day, discard the supernatant, and wash the tube 3 times quickly with PBS; 2% MPBS (containing 2% degreased Milk PBS), blocked at 37°C for 2 hours; discarded the blocking solution, and washed the tube 3 times with PBS quickly; the phage antibody library (10 12 ~10 13 p.f.u) was suspended in 4ml 2% MPBS and added to the immunotube. After repeated inversion at room temperature for 30 minutes, it was left to stand at room temperature for more than 90 minutes; the tube was washed 10 times with PBS containing 0.1% Tween-20, and then washed 10 times with PBS to remove Remove detergent; add 1ml 100mM triethylamine (700μl 7.18mol / L triethylamine added to 50ml water), and incubate repeatedly at room temperature for 10min for specific elution; 0.5ml 1M Tris (pH7...
Embodiment 2
[0017] Example 2 Construction of prokaryotic expression plasmid pET22b-F6
[0018] The F6 gene or pET22b vector was double digested with Nco I and Not I (purchased from Takara), and the reaction system was as follows:
[0019]
[0020] The above reaction system was reacted at 37°C for 2h, electrophoresed on 1.0% agarose, recovered by cutting the gel, dissolved in TE of the sample, and stored at -20°C for future use.
[0021] The double digested F6 gene and pET22b vector were ligated with T4 ligase (purchased from Takara), and the reaction system was as follows:
[0022]
[0023] The above reaction system was reacted at 16°C for 12h.
[0024] Take 10 μl of the constructed plasmid pET22b-F6, add it to 90 μl of competent cell (BL21) suspension, and mix thoroughly; after placing it in an ice bath for 30 minutes, immediately place it in a 42°C water bath for heat shock for 45 seconds, and then place it in an ice bath for 2 minutes; Add 900μl fresh LB liquid medium, shake an...
Embodiment 3
[0025] Example 3 Soluble expression and isolation and purification of anti-hTNF-α single-chain antibody
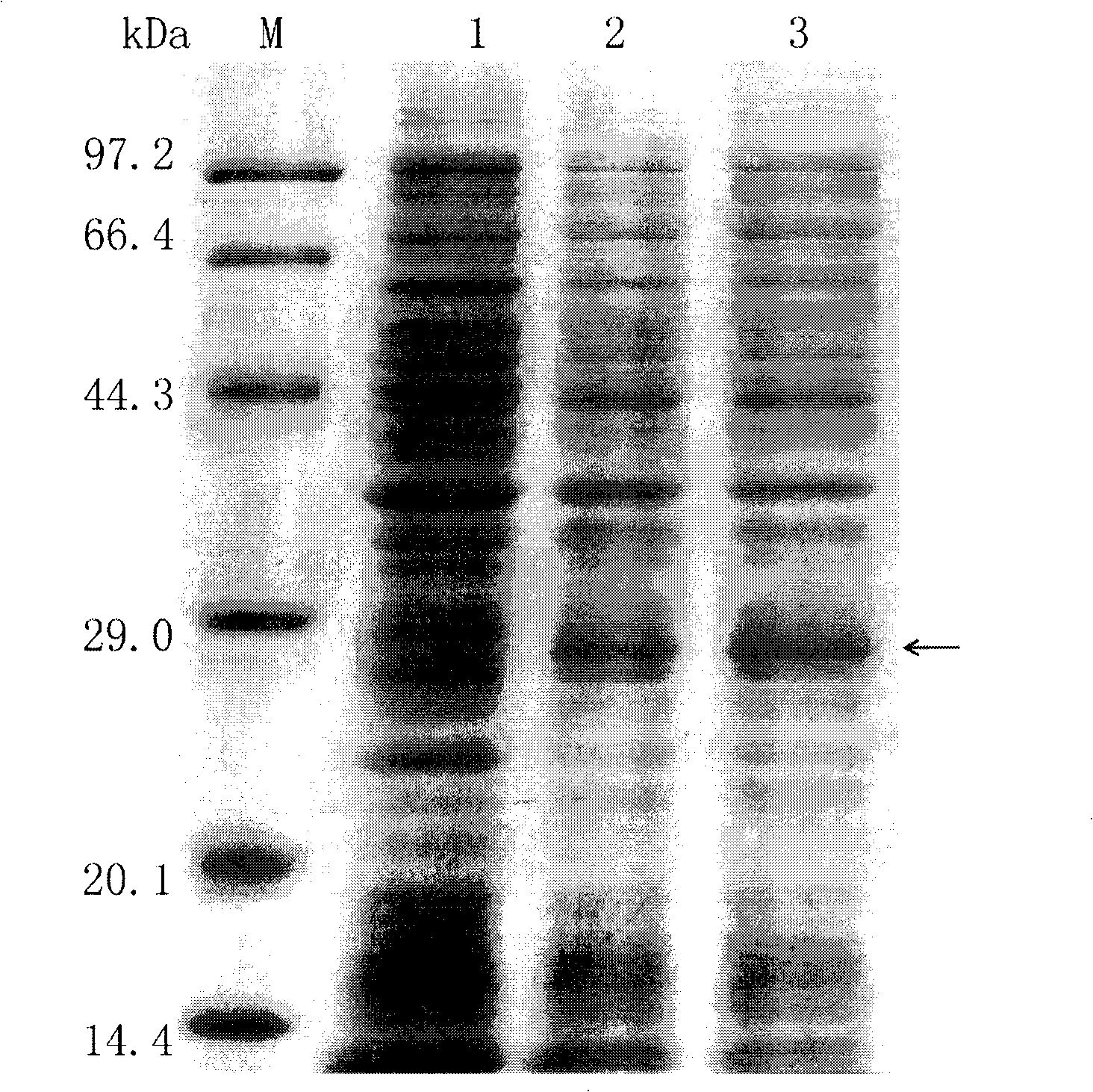
[0026] Select the strain with correct sequencing and culture to OD 600 ≈0.6, add IPTG with a final concentration of 1mM, induce overnight at 37°C, and detect the result of induced expression by 12% SDS-PAGE ( image 3 ). The band indicated by the arrow is the target protein induced to express, and the band size is about 28kDa, which is consistent with the theoretical value.
[0027] 6000rpm / min, centrifuge at 4°C for 5min, collect the cells; resuspend the cells in PBS, sonicate (sonication 3s, interval 3s, 10min in total); 10000rpm / min, centrifuge at 4°C for 20min, keep the supernatant; pass the supernatant over nickel column (Novagen product), elution with different concentrations of imidazole; 12% SDS-PAGE detection of purified results ( Figure 4 ), store the target protein at -20°C. Figure 4 The size of the target band indicated by the middle arrow is about 28kDa,...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com