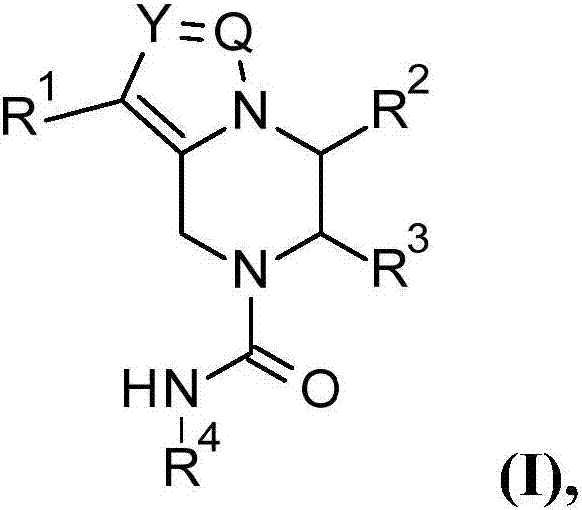
Pyrazine compounds for the treatment of infectious diseases
A compound and alkyl technology, applied in anti-infective drugs, drug combination, organic chemistry, etc., can solve the problems that cannot be realized
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0985] 3-(4-fluorophenyl)-N-phenyl-6,7-dihydro-4H-pyrazolo[1,5-a]pyrazine-5-carboxamide
[0986]
[0987] The target compound was prepared according to the following scheme:
[0988]
[0989] Step 1: Preparation of tert-butyl N-(2-hydroxyethyl)-N-(1H-pyrazol-5-ylmethyl)carbamate (Compound 1b)
[0990] To a solution of 1H-pyrazole-5-carbaldehyde (Compound 1a, 54.0 g, 562.5 mmol) in MeOH (300 mL) was added 2-aminoethanol (41.2 g, 675 mmol), and the reaction mixture was stirred at 25°C for 1 hour. Then add NaBH at 0°C 4 (25.9 g, 675.0 mmol), the reaction mixture was stirred for an additional 1 hour. Add H to the reaction mixture 2 O (300mL) and Boc 2 O (147.1 g, 675.0 mmol), then the obtained mixture was stirred at room temperature for 12 hours, extracted with EtOAc (600 mL). The organic layer was dried over sodium sulfate, filtered and concentrated. The residue was purified by column chromatography (eluting with 20%-50% EtOAc in petroleum ether) to obtain compound 1b...
Embodiment 2
[1004] N,3-Diphenyl-6,7-dihydro-4H-pyrazolo[1,5-a]pyrazine-5-carboxamide
[1005]
[1006] The preparation of embodiment 2:
[1007] The title compound was prepared in a similar manner to Preparation Example 1, using phenylboronic acid instead of (4-fluorophenyl)boronic acid. Example 2 was obtained as a white solid (30 mg). LCMS (M+H + ): 319. 1 H NMR (400MHz, DMSO-d 6 )δppm 8.90(s,1H),7.85(s,1H),7.51-7.38(m,6H),7.30-7.20(m,3H),6.97(m,1H),4.95(s,2H),4.24( m,2H), 4.01(m,2H).
Embodiment 3
[1009] 3-(3-Fluorophenyl)-N-phenyl-6,7-dihydro-4H-pyrazolo[1,5-a]pyrazine-5-carboxamide
[1010]
[1011] The preparation of embodiment 3:
[1012] The title compound was prepared in a similar manner to Preparation Example 1, using (3-fluorophenyl)boronic acid instead of (4-fluorophenyl)boronic acid. Example 3 was obtained as a white solid. LCMS (M+H + ): 337.1H NMR (400MHz, chloroform-d) δppm 7.75(s,1H),7.46-7.31(m,5H),7.18-6.97(m,4H),6.47(s,1H),4.91(s,2H ), 4.37(t, J=5.3Hz, 2H), 4.06(t, J=5.4Hz, 2H).
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com