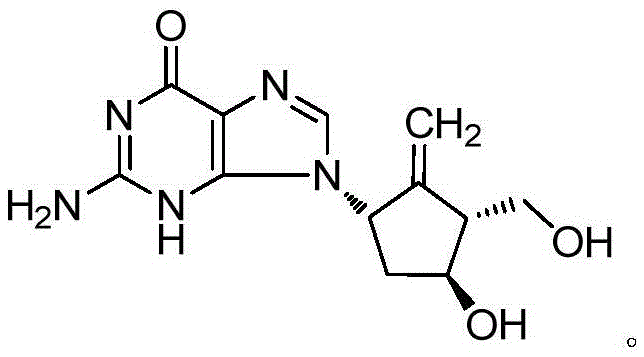
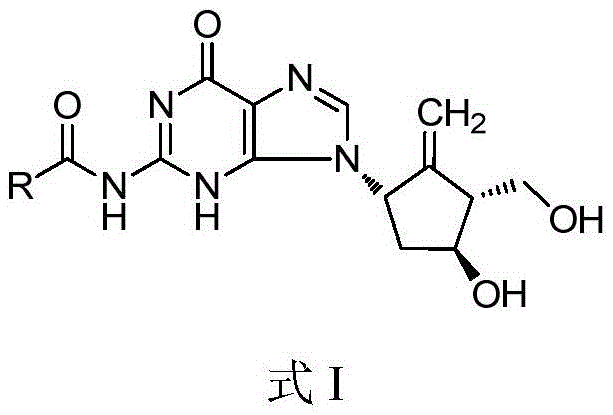
Entecavir fatty acid derivatives and pharmaceutical composition thereof
A fatty acid derivative, entecavir technology, applied in the field of medicine, can solve the problems of high solubility adverse reactions, hindering transmembrane transport, and low bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
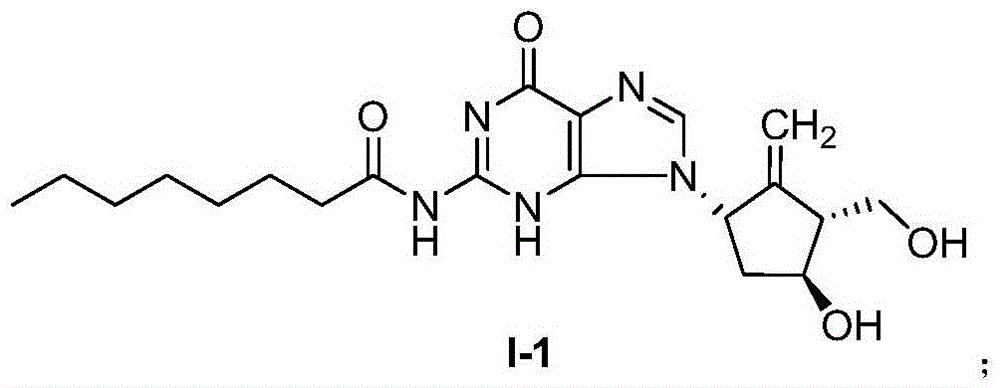
[0033] Example 1: N-1,9-dihydro-9-[(1S,3R,4S)-4-hydroxy-3-hydroxymethyl-2-methylenecyclopentyl]-6H-purine-6- The preparation of keto-2-octanoamide (formula I-1, entecavir-2-octanoamide)
[0034]
[0035] 1.0 g of entecavir was dissolved in 20 mL of dichloromethane, and 587 mg of caprylic acid chloride was added in an equimolar amount. After mixing, 0.5 mL of pyridine was added, and the reaction was carried out at 30° C. for 8 hours. After the reaction, dichloromethane was distilled off, 10mL of water was added to the obtained crude product, and reacted at 30°C for 0.5 hours to eliminate unreacted caprylic acid chloride, then 100mL of water was added, after mixing, extracted with dichloromethane, and the organic phase was washed with anhydrous Sodium sulfate to remove residual moisture, filter, and distill methylene chloride under reduced pressure to obtain N-1,9-dihydro-9-[(1S,3R,4S)-4-hydroxyl-3-hydroxymethyl-2- Methylenepentyl]-6H-purin-6-one-2-octanamide (1.17 g, 74%). ...
Embodiment 2
[0037] Example 2: N-1,9-dihydro-9-[(1S,3R,4S)-4-hydroxy-3-hydroxymethyl-2-methylenecyclopentyl]-6H-purine-6- The preparation of ketone-2-lauroylamide (formula I-2, entecavir-2-lauroylamide)
[0038]
[0039] 1.0 g of entecavir was dissolved in 20 mL of dichloromethane, and 790 mg of lauric acid chloride was added in an equimolar amount. After mixing, 0.5 mL of pyridine was added and reacted at 50°C for 8 hours. After the reaction, dichloromethane was distilled off, 10mL of water was added to the resultant, and reacted at 30°C for 0.5 hours to eliminate unreacted lauric acid chloride, then 100mL of water was added, after mixing, extracted with dichloromethane, and the organic phase was Use anhydrous sodium sulfate to remove residual moisture, filter, and distill off dichloromethane under reduced pressure to obtain N-1,9-dihydro-9-[(1S,3R,4S)-4-hydroxy-3-hydroxymethyl -2-Methylenepentyl]-6H-purin-6-one-2-lauramide (1.22 g, 68%).
[0040] MS(ES):460.34(M+H + )
Embodiment 3
[0041] Embodiment 3: the preparation of entecavir-2-octanoamide tablet (1000)
[0042]
[0043] The above materials were crushed separately, sieved with 8 meshes, after entecavir-2-octylamide, microcrystalline cellulose and starch were fully mixed, an appropriate amount of starch slurry was added to make a soft material, sieved with 12 meshes, dried at 50°C for 2 hours, and added Sodium carboxymethyl starch and magnesium stearate are mixed evenly, and pressed into tablets to obtain the product.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com