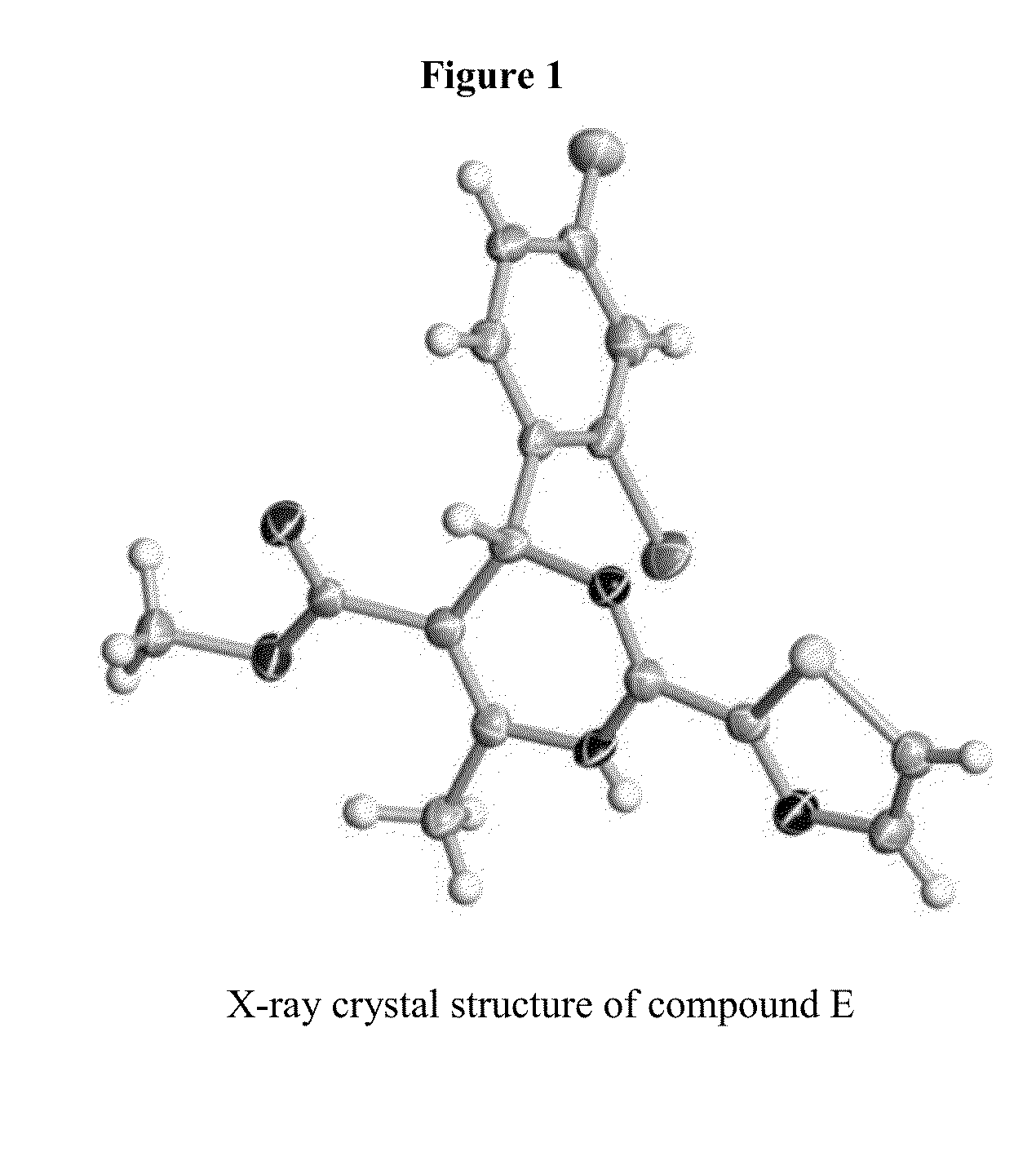
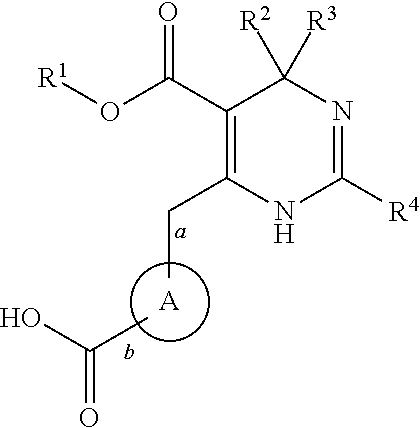
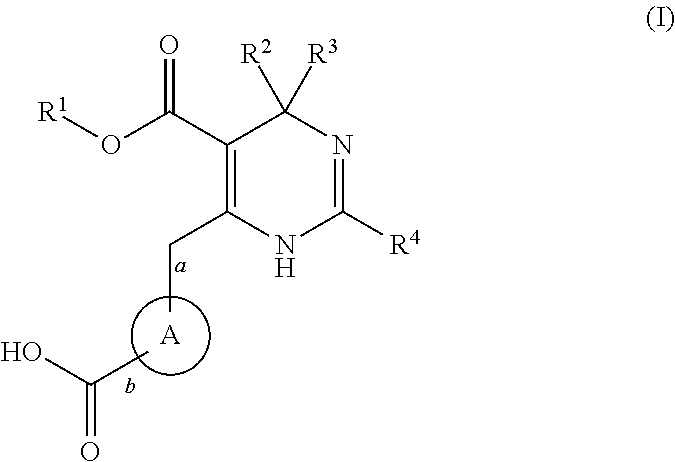
Novel 6-amino acid heteroaryldihydropyrimidines for the treatment and prophylaxis of hepatitis b virus infection
a technology of hepatitis b virus and pyrimidine, which is applied in the field of new 6-amino acid heteroaryldihydropyrimidines for the treatment and prophylaxis of hbv infection, can solve the problems of poor long-term response and debilitating side effects, viral genotypes that do not show good responses to interferon therapy, and serious public health problems such as hbv, and achieve superior anti-hbv activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1 and 2
[0308]6-((S)-2-Carboxy-4,4-difluoro-pyrrolidin-1-ylmethyl)-4-(2-chloro-4-fluoro-phenyl)-2-thiazol-2-yl-1,4-dihydro-pyrimidine-5-carboxylic acid methyl ester (Example 1) and (R)-6-((S)-2-carboxy-4,4-difluoro-pyrrolidin-1-ylmethyl)-4-(2-chloro-4-fluoro-phenyl)-2-thiazol-2-yl-1,4-dihydro-pyrimidine-5-carboxylic acid methyl ester (Example 2)
Procedure A
[0309]The title compounds were prepared according to the general synthetic routes shown in Scheme 1 and Scheme 3. A detailed synthetic route is provided in Scheme 4.
Preparation of Compound A1
[0310]To a stirred solution of thiazole-2-carbonitrile (1.5 g, 14 mmol) in 5 mL of dry MeOH was added dropwise a solution of sodium methoxide (0.74 g, 14 mmol) in 10 mL of dry methanol. The reaction mixture was stirred at room temperature until the disappearance of starting material which was checked by LC / MS. After that, ammonium chloride (1.5 g, 28 mmol) was added in one portion and the reaction mixture was stirred overnight. The undissolved materia...
preparation of example 1
[0313]To a stirred solution of 6-bromomethyl-4-(2-chloro-4-fluoro-phenyl)-2-thiazol-2-yl-1,4-dihydro-pyrimidine-5-carboxylic acid methyl ester (0.049 g, 0.11 mmol) and (S)-4,4-difluoro-pyrrolidine-2-carboxylic acid (0.044 g, 0.17 mmol) in 1,2-dichloroethane (5 mL) was added dropwise DIPEA (0.078 mL, 0.45 mmol). The reaction mixture was stirred at room temperature until the disappearance of starting material which was checked by LC / MS. The mixture was diluted with EtOAc (50 mL) and washed successively with saturated aqueous NH4Cl solution and brine. The organic layer was separated and dried over Na2SO4. The solvent was concentrated in vacuo and the crude product was purified by prep-HPLC to give 6-((S)-2-carboxy-4,4-difluoro-pyrrolidin-1-ylmethyl)-4-(2-chloro-4-fluoro-phenyl)-2-thiazol-2-yl-1,4-dihydro-pyrimidine-5-carboxylic acid methyl ester (Example 1).
Preparation of Example 2
[0314]The enantiopure (R)-6-((S)-2-carboxy-4,4-difluoro-pyrrolidin-1-ylmethyl)-4-(2-chloro-4-fluoro-phenyl...
preparation of example 2
[0320]To a stirred solution of (R)-6-bromomethyl-4-(2-chloro-4-fluoro-phenyl)-2-thiazol-2-yl-1,4-dihydro-pyrimidine-5-carboxylic acid methyl ester (0.049 g, 0.11 mmol) and (S)-4,4-difluoro-pyrrolidine-2-carboxylic acid (0.044 g, 0.17 mmol) in 1,2-dichloroethane (5 mL) was added dropwise DIPEA (0.078 mL, 0.45 mmol). The reaction mixture was stirred at room temperature until the disappearance of starting material which was checked by LC / MS. The mixture was diluted with EtOAc (50 mL) and washed successively with saturated aqueous NH4Cl solution and brine. The organic layer was separated and dried over Na2SO4. The solvent was concentrated in vacuo and the crude product was purified by prep-HPLC to give (R)-6-((S)-2-carboxy-4,4-difluoro-pyrrolidin-1-ylmethyl)-4-(2-chloro-4-fluoro-phenyl)-2-thiazol-2-yl-1,4-dihydro-pyrimidine-5-carboxylic acid methyl ester (Example 2) as a light yellow solid.
PUM
| Property | Measurement | Unit |
|---|---|---|
| pH | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| pressure | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com