A kind of antiviral medicine and preparation method thereof
A drug and viral technology, which is applied to the ester of peramivir and plerixafor-N-alkanoic acid, the derivative ester of peramivir, and the preparation field of the antiviral drug, which can prolong the survival time, The effect of increased mortality protection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
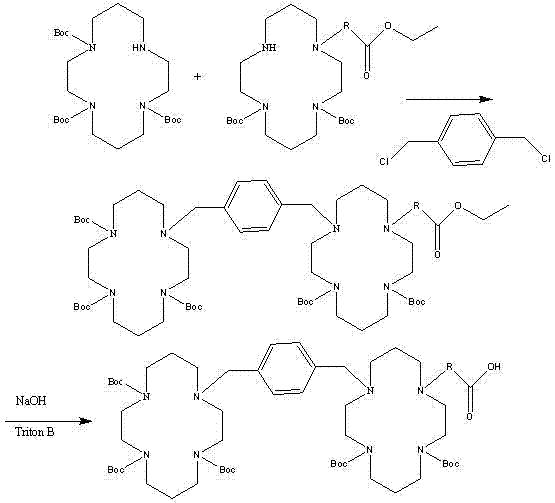
[0046] Example 1 (1S,2S,3R,4R)-1'-carboxylic acid-3'-[(1S)-1''-acetamido-2''-ethyl]butyl-4'-guanidino Cyclopenta-2'-oxo-{5-[1,4,8,11-tetraazacyclotetradecyl]methyl}phenyl)-1,4,8,11-tetraazacyclotetradecyl Alkyl]}-valerate
[0047]
[0048] (1) (1S,2S,3R,4R)-2-Hydroxy-3-[(1S)-1-acetamido-2-ethyl]butyl-4-diBoc-guanidinocyclopenta-1- Benzyl carboxylate
[0049]
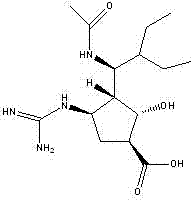
[0050] Add 32.8g (100mmol) of peramivir, 21.3g (112mmol) of p-toluenesulfonic acid, 54.1g (500mmol) of benzyl alcohol, and add 500mL of toluene to reflux for 24 hours (using a water separator). After the reaction is completed, Add ethyl acetate to freeze and crystallize, suction filter, wash with ethyl acetate, add 200mL tetrahydrofuran to the filter cake to dissolve, add 30.3g (300mol) of triethylamine, add 109.1g (500mmol) of di-tert-butyl dicarbonate (BOC anhydride ), stirred at room temperature for 24 hours, added 400 mL of dichloromethane after the reaction was completed, adjusted the pH to 2-3 with hydroch...
Embodiment 2
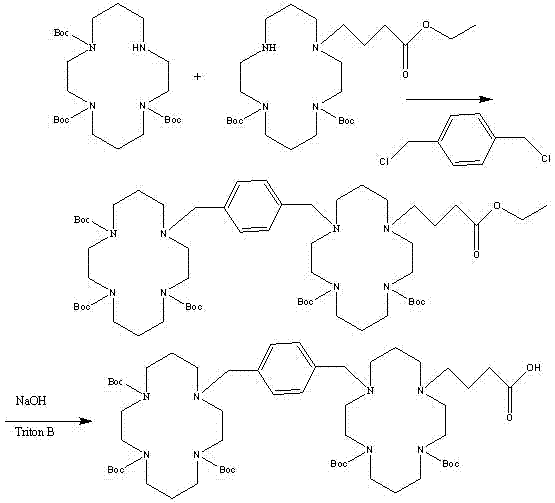
[0066] Example 2 (1S,2S,3R,4R)-1'-carboxylic acid-3'-[(1S)-1''-acetamido-2''-ethyl]butyl-4'-guanidino Cyclopenta-2'-oxo-{5-[1,4,8,11-tetraazacyclotetradecyl]methyl}phenyl)-1,4,8,11-tetraazacyclotetradecyl Alkyl]}-octadecanoate
[0067]
[0068] Prepared as in Example 1, replacing ethyl 5-bromopentanoate in step (3) with ethyl 18-bromo-octadecanoate to obtain the target compound; high-resolution mass spectrum (+ESI, m / z): 1096.6419 [M+H] + .
Embodiment 3
[0069]Example 3 (1S,2S,3R,4R)-1'-carboxylic acid-3'-[(1S)-1''-acetamido-2''-ethyl]butyl-4'-guanidino Cyclopenta-2'-oxo-{5-[1,4,8,11-tetraazacyclotetradecyl]methyl}phenyl)-1,4,8,11-tetraazacyclotetradecyl Alkyl]}-methyl-phenyl-valerate
[0070]
[0071] Prepared as in Example 1, replacing ethyl 5-bromovalerate in step (3) with p-bromomethyl-ethyl valerate to obtain the target compound; high-resolution mass spectrum (+ESI, m / z): 1004.4098 [M+H] + .
[0072] The selectivity index and the death protection rate of the peramivir derivative ester provided by the present invention are further compared and illustrated by the test examples below.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com