A kind of indole derivative and its use
A technology for indole derivatives and drugs, applied to indole derivatives and their application fields, can solve the problems of low oral bioavailability, low toxicity and side effects, respiratory and nervous system disorders, etc., and achieves small cytotoxicity, high selectivity index, Highly active effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
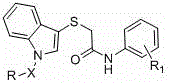
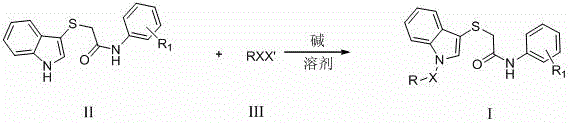
[0029] Embodiment 1: Intermediate 2-((1 H -Indol-3-yl)-mercapto)- N -The synthesis of (2-bromophenyl) acetamide, concrete operation is as follows:
[0030]
[0031] Add 3-mercaptoindole (0.01mol) into a dry three-necked bottle, use 20ml N,N -After dissolving dimethylformamide (DMF), add anhydrous potassium carbonate solid (0.01mol), stir for a while, add 2-bromo- N -(2-Bromophenyl)acetamide (0.01mol), heated to 80°C and stirred for 5 hours, TLC tracked the reaction until the raw material point disappeared, stopped the reaction, poured the reaction solution into ice water and stirred to precipitate a precipitate, pumped filtered, washed with ice water, and dried to obtain 2-((1 H -Indol-3-yl)-mercapto)- N The crude -(2-bromophenyl)acetamide was directly used in the next step without purification.
Embodiment 2
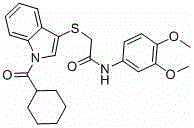
[0032] Example 2: N -(3,4-Dimethoxyphenyl)-2-((1-(cyclohexylmethyl)-1 H - the synthesis of indol-3-yl) mercapto) acetamide (Ia), concrete operation is as follows:
[0033] Add 2-((1 H -Indol-3-yl)mercapto)- N -(3,4-dimethoxyphenyl)acetamide (2mmol), with 3mL N,N -After dimethylformamide (DMF) dissolves, add NaH (3mmol, 0.072g) three times under stirring condition in an ice bath, stir for a while, add bromomethylcyclohexane (3mmol, 0.5312g), stir for 3 Hour. The reaction was followed by TLC until the raw material point disappeared, and the reaction was stopped. The reaction solution was poured into ice water, extracted three times with ethyl acetate, and the ethyl acetate was distilled off under reduced pressure to obtain a crude product. The crude product was purified by column chromatography (petroleum ether: ethyl acetate = 15:1) to obtain the pure product of white powder solid Ia, and the detection results are as follows:
[0034]
[0035] Yield: 68.0%; Melting poi...
Embodiment 3
[0037] Example 3: N -(2-Bromophenyl)-2-((1-(cyclohexylmethyl)-1 H - the synthesis of indol-3-yl) mercapto) acetamide (Ib), concrete operation is as follows:
[0038]Add 2-((1 H -Indol-3-yl)mercapto)- N -(2-Bromophenyl)acetamide (2mmol), with 3mL N,N - After dimethylformamide (DMF) was dissolved, NaH (3mmol, 0.072g) was added three times under the condition of stirring in an ice bath, stirred for a while, bromomethylcyclohexane (3mmol, 0.5312g) was added, stirred for 5 Hour. The reaction was followed by TLC until the raw material point disappeared, and the reaction was stopped. The reaction solution was poured into ice water, extracted three times with ethyl acetate, and the ethyl acetate was distilled off under reduced pressure to obtain a crude product. The crude product was purified by column chromatography (petroleum ether: ethyl acetate = 15:1) to obtain pure white powder solid Ib. The test results are as follows:
[0039]
[0040] Ib
[0041] Yield: 73.5% Melti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com