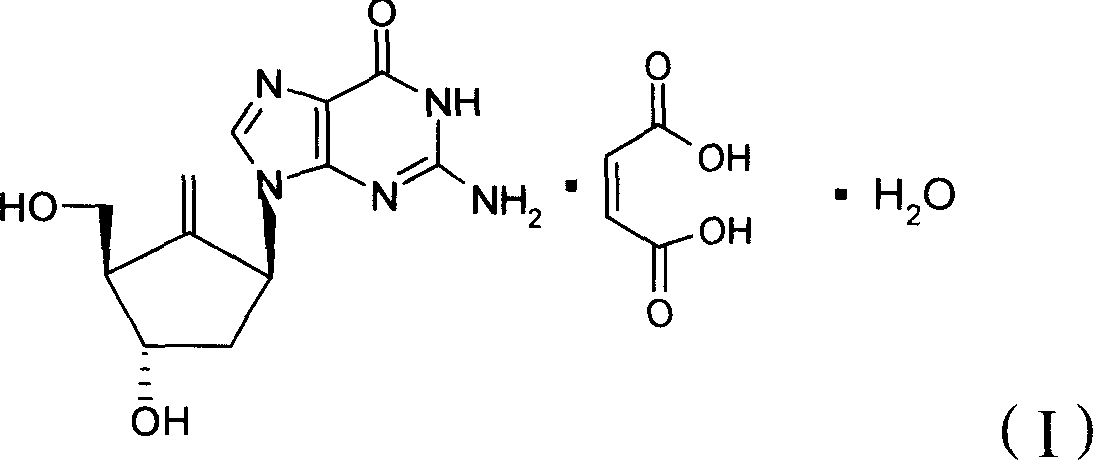
Process for preparing purine derivatives
A technology for derivatives and purines, applied in the field of preparing purine derivatives, can solve the problems of unsuitable large-scale preparation of entecavir raw materials, complicated preparation process and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0056] 1) Preparation of Intermediate A
[0057]
[0058] Put 500ml of dry tetrahydrofuran in a 2000ml three-necked flask, and cool it to about -75°C with liquid nitrogen under the protection of nitrogen, and add 116.0g (0.68mol) of 4-methyl-benzylchloromethyl to the reaction flask ether. Put 392ml (0.69mol) cyclopentadiene sodium tetrahydrofuran solution in the balance funnel, then slowly add it dropwise into the reaction flask, control the reaction temperature at -60~-50°C, and continue to stir at this temperature for 1 hours, the intermediate A was directly used in the next reaction.
[0059] 2) Preparation of intermediate (1)
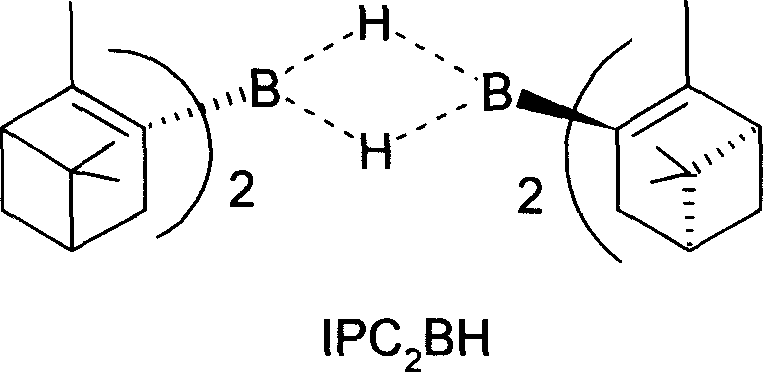
[0060] Preparation of IPC2BH
[0061]
[0062] Draw 800.0 ml (0.80 mol) of borane tetrahydrofuran solution (1.0 mol) with a syringe, and place it in an accurately weighed 250 ml two-necked round bottom flask equipped with a -80°C thermometer and a stirring bar. Under the protection of N2, cool in an ice bath to 0°C, extract 160.0ml (0.902m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com