Application of interferon-induced transmembrane protein 3 (IFITM 3) for preparing medicament against hepatitis B virus (HBV) infection
A hepatitis B virus and protein technology, applied in antiviral agents, drug combinations, peptide/protein components, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
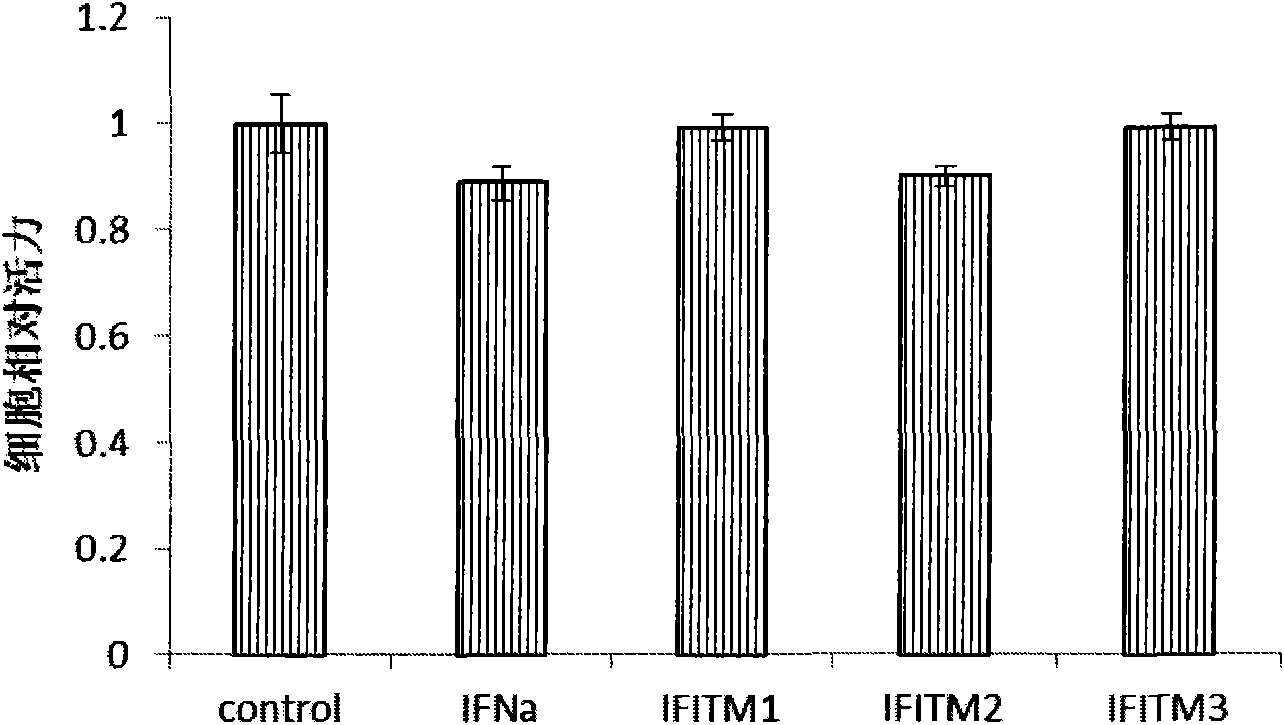
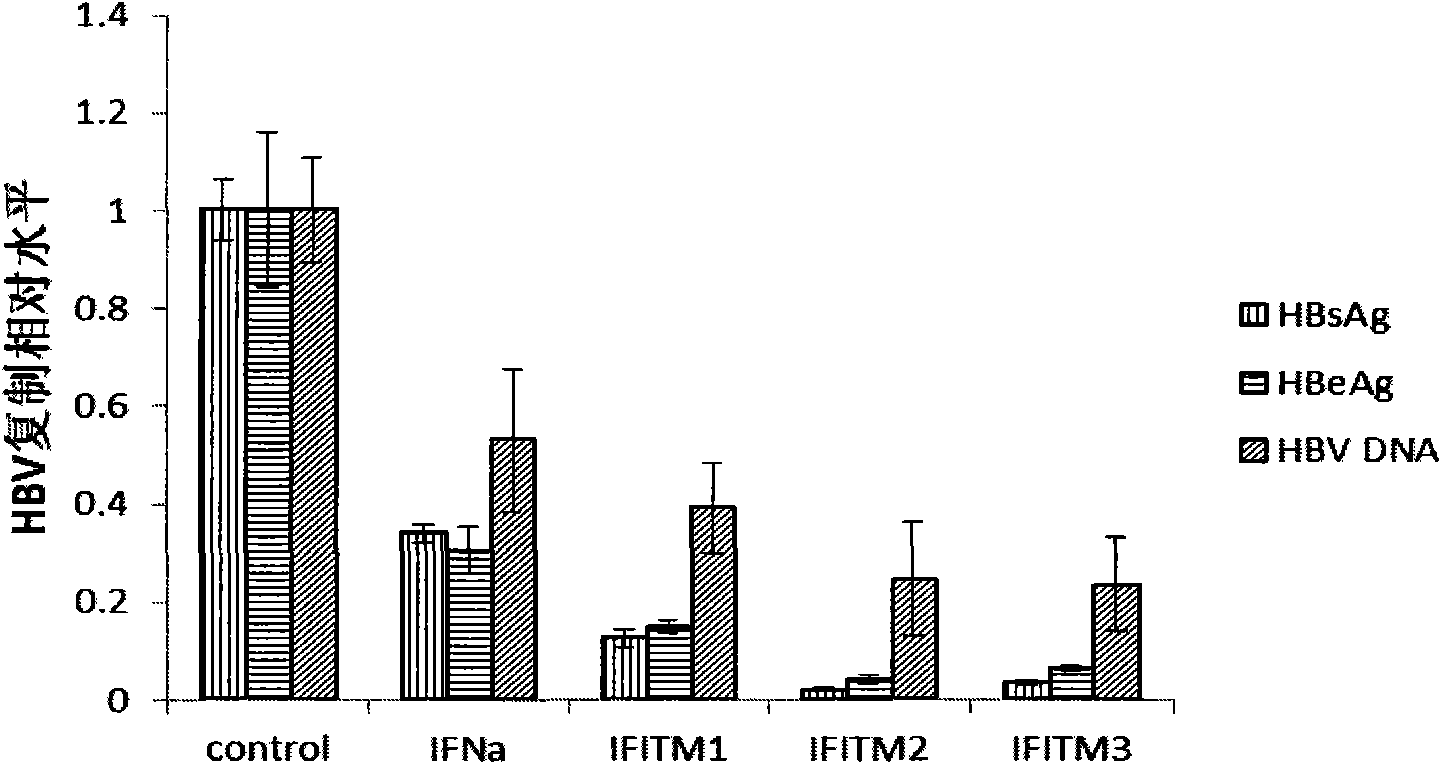
[0042] Example 1 Intracellular overexpression of IFITMs protein molecules inhibits HBV replication
[0043] Materials and Methods
[0044] 1. Cell culture
[0045] The cells used were hepatoma cells HepG2, which were cultured in DMEM containing 10% fetal bovine serum (FBS, Gibco) at 37°C and 5% CO 2 cultivated under conditions.
[0046] 2. Plasmid
[0047] The pAAVMCS plasmid (Stratagene Company) was preserved by our laboratory. Primers were designed according to the CDS region sequences of each protein of IFNa and IFITMs published by NCBI, and inserted into the pAAVMCS plasmid by amplification and enzyme digestion. After positive clone PCR identification, sequencing, and BLAST comparison, the results showed that pAAVMCS-IFNa, pAAVMCS- IFITM1, pAAVMCS-IFITM2 and pAAVMCS-IFITM3 were constructed successfully. The HBV replication plasmid pHBV1.31 contains 1.31 copies of HBV DNA and has a complete replication unit, which can complete virus replication in transfected HepG2 cel...
Embodiment 2
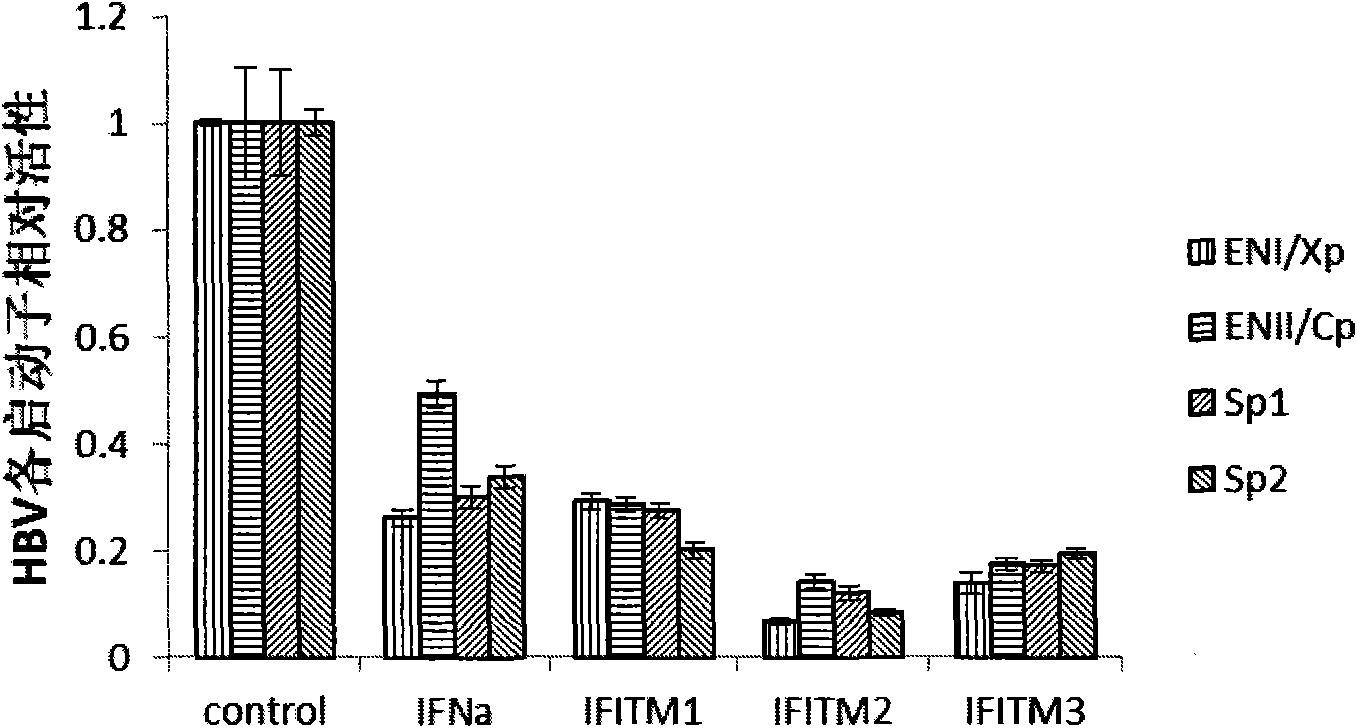
[0060] Example 2 Intracellular overexpression of IFITMs protein molecules inhibits HBV promoter transcriptional activity
[0061] Materials and Methods
[0062] 1. Cell culture
[0063] Method is the same as embodiment one.
[0064] 2. Plasmid
[0065] Gaussia secreted luciferase expression plasmids pENI / Xp-Gluc, pENII / Cp-Gluc, pSp1-Gluc and pSp2-Gluc, which are transcriptionally regulated by different promoters of HBV, were constructed and preserved by our laboratory.
[0066] 3. Cell Transfection
[0067] Basic method is the same as embodiment one. Co-transfect HepG2 cells with pAAVMCS, pAAVMCS-IFNa, pAAVMCS-IFITM1, pAAVMCS-IFITM2, and pAAVMCS-IFITM3, respectively, with equal amounts of pENI / Xp-Gluc, pENII / Cp-Gluc, pSp1-Gluc, and pSp2-Gluc, and transfect After 48 hours, the cell supernatant was collected for immediate detection or stored at -20°C for later use.
[0068] 4. Gaussia luciferase activity detection
[0069] NEB company's Gaussia secreted luciferase detecti...
Embodiment 3
[0074] Example 3 Overexpression of IFITM3 protein molecules in mice significantly inhibits the expression of HBV genes
[0075] Materials and Methods
[0076] 1. Animal modeling
[0077] SPF grade C57 / BL6 mice, 18±2g, were purchased from Beijing Weitong Lihua Co., Ltd. The model was established by rapid injection of large-capacity plasmids into the tail vein of mice, and 10 μg of pAAVMCS, pAAVMCS-IFNα, pAAVMCS-IFITM1, pAAVMCS-IFITM2, pAAVMCS-IFITM3 and 10 μg of pHBV1.18 (containing 1.18 copies of HBV DNA) were co-infected. Dissolve in PBS of 1 / 10 volume of mouse body weight, and inject at a uniform speed within 5-8 seconds.
[0078] 2. Detection of HBsAg and HBeAg
[0079] On the 1st, 3rd, 5th, and 7th day after modeling, blood was taken from the tail vein of the mouse, and after appropriate dilution, HBsAg and HBeAg were detected according to the operation steps of the ELISA quantitative detection kit (Zhengzhou Antu Lvke Bioengineering Co., Ltd.). Take 50 μl Serum at a s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com