Specific HBV antibody
A specificity and antibody technology, applied in the direction of antibodies, specific peptides, antiviral agents, etc., can solve the problems of low specific activity, unknown antigen, insufficient supply, etc., achieve high affinity, reduce viral load, and low allergic reaction original effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
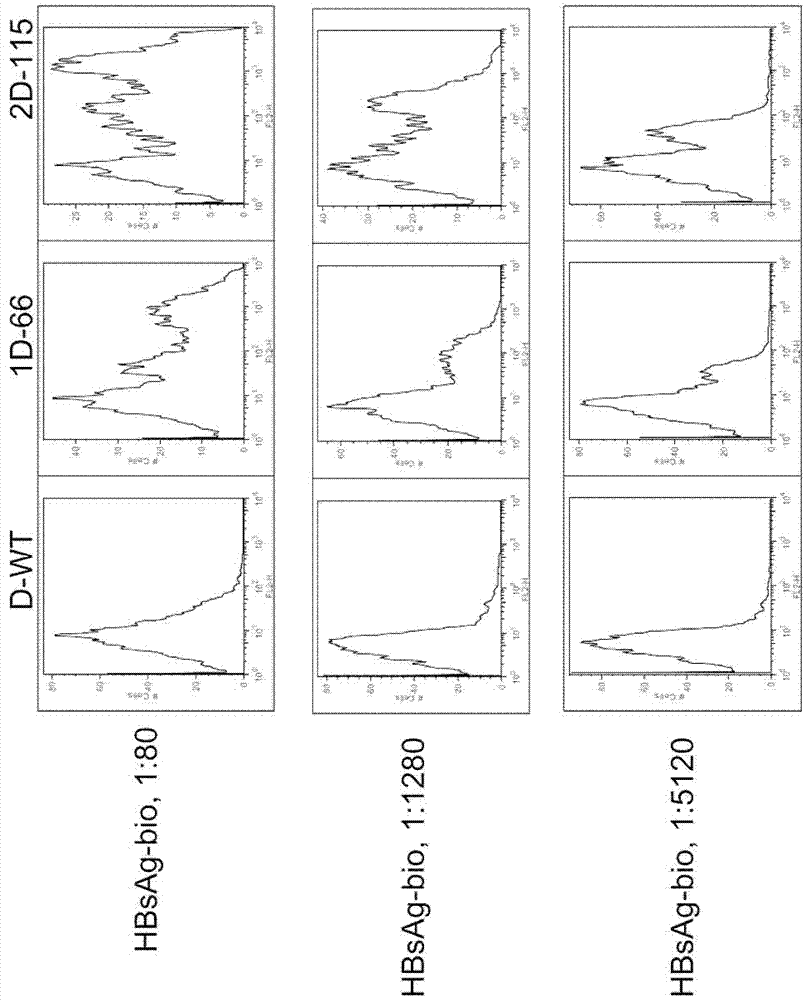
[0111] Example 1 Screening of specific scFv against adr and adw subtype HBsAg
[0112] I. Screening of high-affinity specific scFv
[0113] Using a non-immune human scFv library (see Nature Protocols Vol. 1 No. 2: 755-768, 2006 for specific information about this library), the scFv against adr and adw subtype HBsAg was screened by yeast display method. After the above screening, the inventors isolated and cloned specific single-chain antibodies scFvC (Wt) (SEQ ID NO: 1) and scFvD (Wt) with high affinity for adr and adw subtypes of HBsAg.
[0114] II. Further Screening of Mutated High Affinity scFvs
[0115] Since human monoclonal antibodies selected from human scFv yeast libraries usually have low expression levels, they are difficult to be prepared in an efficient manner and used for large-scale clinical applications. To overcome this problem, the above screened human scFvs of HBsAg specific to adr and adw subtypes (ie, scFvC and scFvD) can be further mutated. Taking s...
Embodiment 2
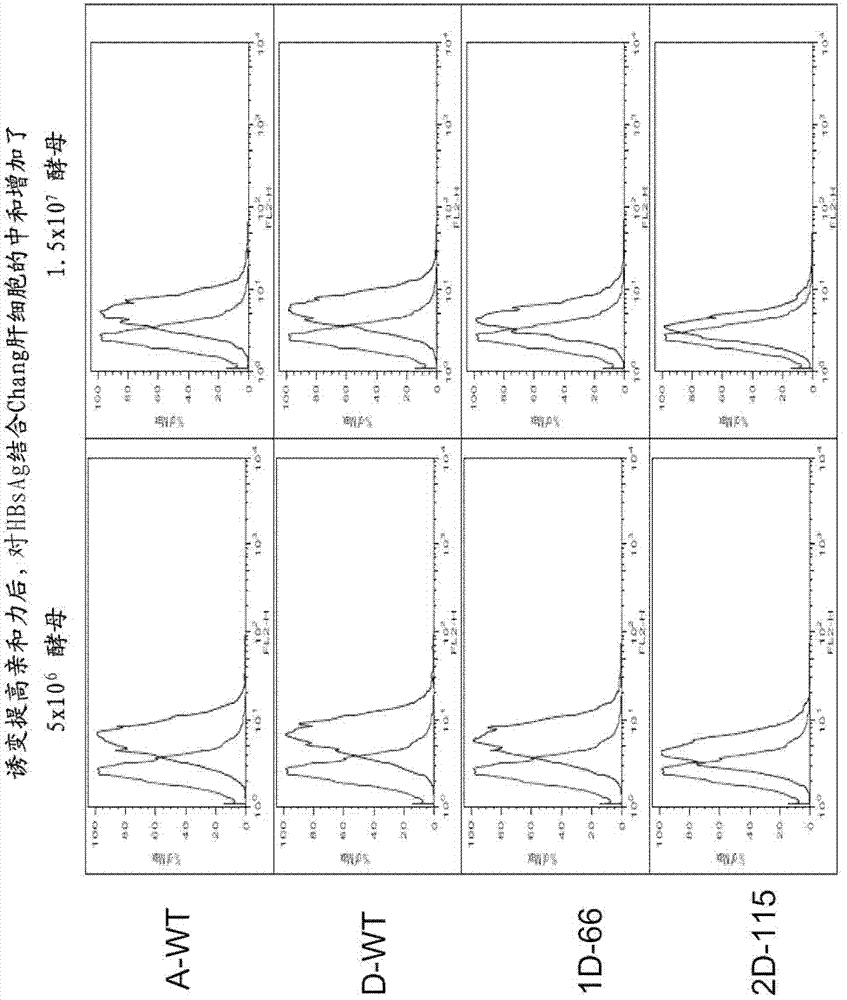
[0116] scFvD (wt), scFvD (1D-66) screened in Example 2 and scFvD(2D-115) can neutralize the binding of HBsAg to hepatocytes
[0117] In order to detect whether the obtained high-affinity and specific antibody can neutralize the binding of HBsAg to hepatocytes, the inventors detected the ability of the obtained single-chain antibody to specifically neutralize HBsAg. First, the inventor used 100 μl of 5 μg / ml HBsAg-bio(adw) (Shanghai PrimeGene Biotechnology Company, #671-02; in the laboratory, the purchased HBsAg(adw) was biotinylated according to the standard method, diluted to 5 μg / ml) were pre-incubated with yeast cells (5x10) expressing scFvA (A-WT), scFvD (wt), scFvD (1D-66) 6 and 1.5x10 7 cells), and the single-chain antibody scFvA (A-WT) without binding specificity was used as a negative control. The pre-incubation was carried out for 5 minutes at room temperature. With 7000rpm the yeast cells were centrifuged for 3 minutes, the supernatant was taken and mixed with...
Embodiment 3
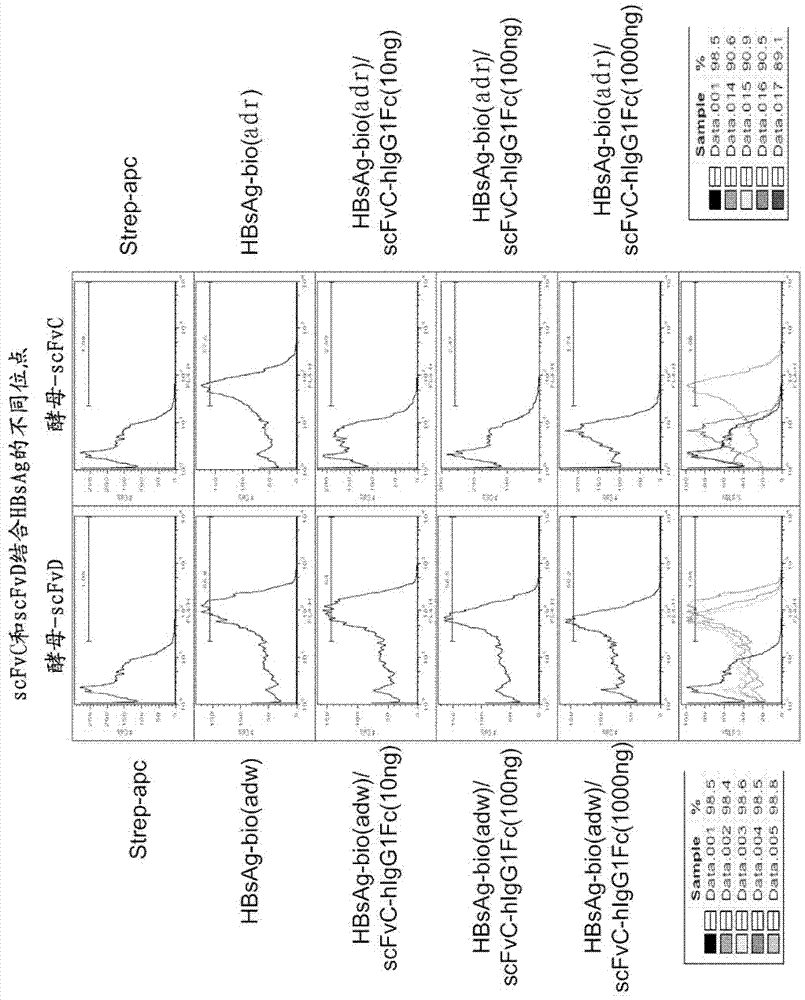
[0119] Example 3 Monoclonal antibodies scFvD-hIgG1Fc and scFvC-hIgG1Fc can complete Total blockage of HBsAg binding to Chang hepatocytes
[0120] In order to further test whether the antibody of the present invention can prevent the binding of the virus to liver cells, according to the method described in reference 7, the inventors used the scFvC (wt) and scFvD (2D-115) screened above to construct monoclonal antibodies respectively scFvC-hIgG1Fc and scFvD-hIgG1Fc. Then, the inventors examined the ability of the scFvC-hIgG1Fc and scFvD-hIgG1Fc to neutralize the binding of HBsAg to Chang hepatocytes.
[0121] In order to detect the ability of scFvD-hIgG1Fc to neutralize the binding of HBsAg to Chang hepatocytes, Chang hepatocytes (5.3×10 5 / ml) placed in the container. Then dilute HBsAg-bio (adw) (Shanghai PrimeGene Biotechnology Company, #671-02) to 5 μg / ml. Add 25 μl of the diluted HBsAg-bio(adw) / 5ml tube to the reaction tube, then add different amounts of each antibody...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com