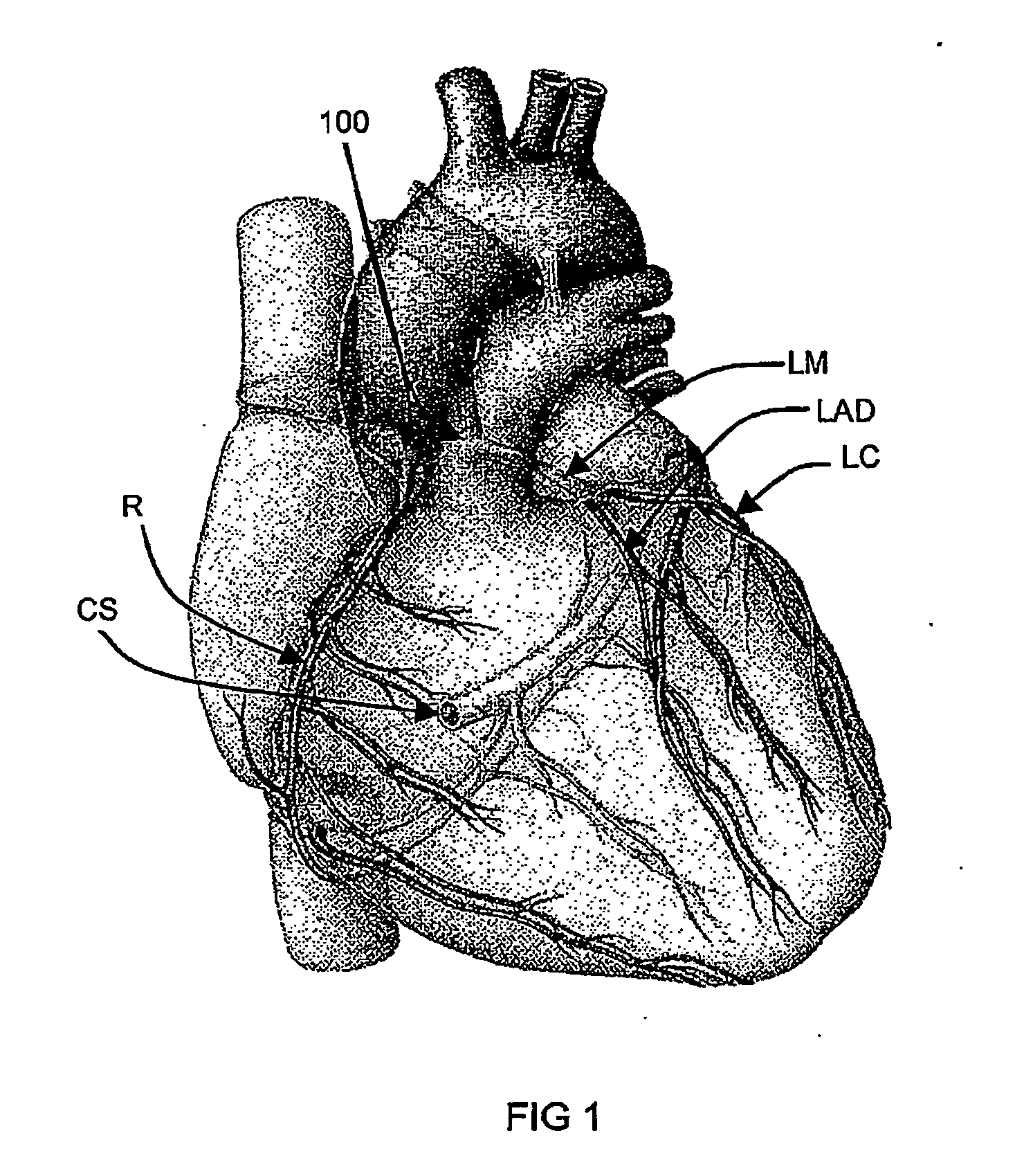
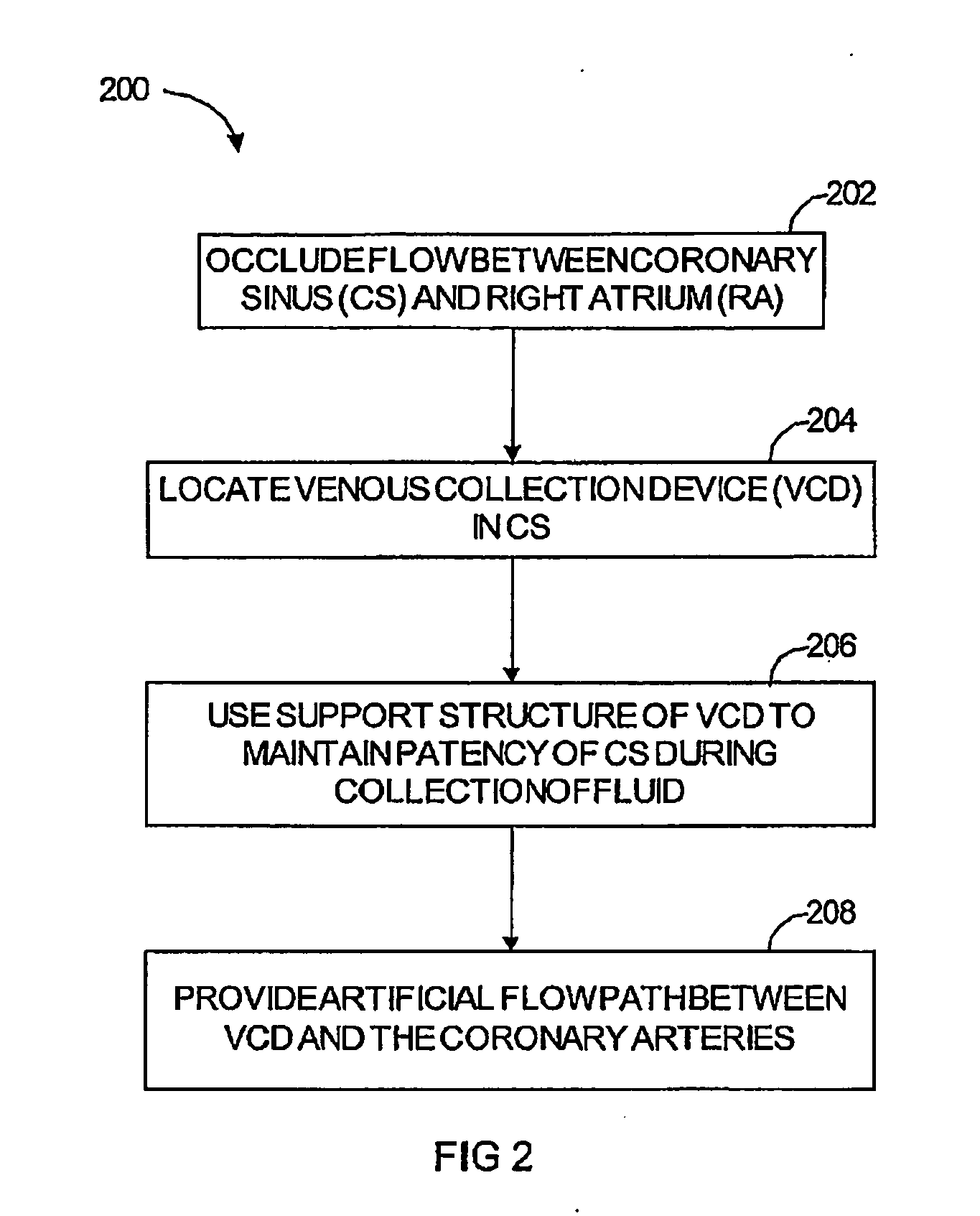
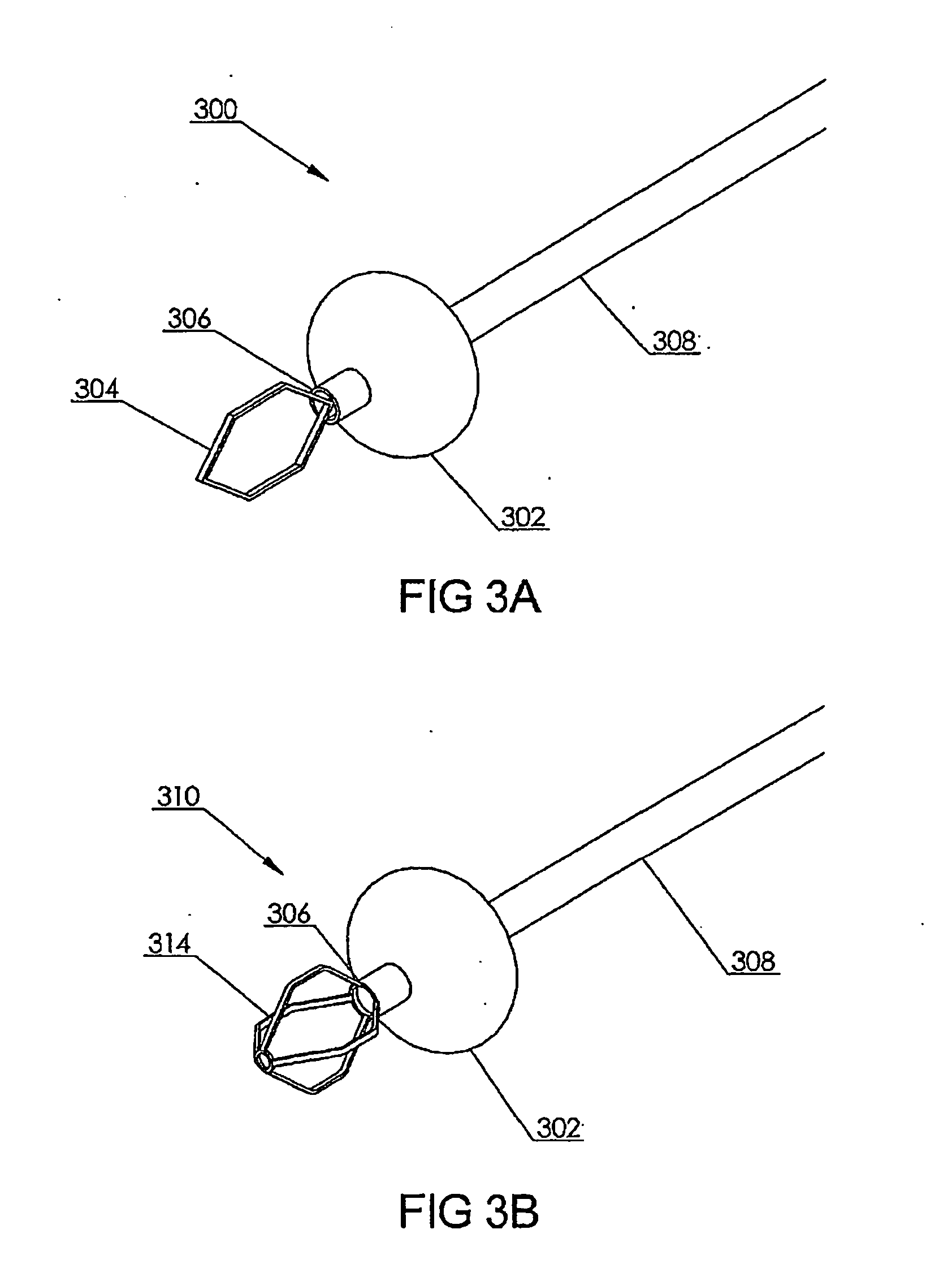
Polynucleotide delivery to cardiac tissue
a polynucleotide and tissue technology, applied in the field of cardiology, can solve the problems of drug degradation, drug degradation, and difficulty in delivering therapeutically active agents to cardiac tissue, and achieve the effect of improving or maintaining the ejection fraction of the cardiovascular system and improving the ejection fraction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Recirculating Delivery of a Pseudophosphorylated Mutant of Phospholamban (S16E) Prevents the Progression of Heart Failure in a Large Animal Model
[0197] Using an embodiment of a cardiac isolation circuit described herein, the coronary venous circulation in a sheep was isolated from the systemic circulation. This isolated cardiac circuit provided a recirculating percutaneous system for the selective delivery of viral vectors to myocardium. Using this system, adenovirus (3.5×1012vp) encoding a pseudophosphorylated mutant of phospholamban was delivered to sheep as described below. The sequence of wild-type human phospholamban (PLN) is obtainable from Genbank under accession number NM—002667, incorporated herein by reference. The pseudophosphorylated mutant used in this study (S16E) is a point mutation of phospholamban at amino acid 16 from S to E.
[0198] Animal Procedures
[0199] Day 0—Cardiac Pacemaker Implantation
[0200] Anesthesia and Site Preparation
[0201] The animal foreleg was cl...
example 2
Delivery of SERCA2a to Cardiac Cell
[0227] Using a the same technique as in Example 1 for isolating the coronary venous circulation and delivering viral vector to sheep myocardium, adenovirus was delivered (3.5×1012vp) encoding the sarcoplasmic reticulum ATPase 2a (AdSERCA2a (n=2)) or adenovirus encoding a control gene for β-Galactosidase (LacZ;AdLacZ (n=6, 4.7×1012vp) to sheep with pacing induced heart failure. The sequence of human SERCA2a can be obtained at Genbank accession numbers NM—170665 and NM—001681, incorporated herein by reference. Despite 2 weeks further pacing, treatment with AdSERCA2a significantly improved contractile function despite ongoing pacing stress and prevented ventricular remodeling in contrast to AdLacZ animals, as shown in FIG. 5.
example 3
Delivery of β-Galactosidase to a Cardiac Cell
[0228] Using the same technique as in Example 1 for isolating the coronary venous circulation and delivering viral vector to sheep myocardium described in Examples 1 and 2, we delivered adeno-associated virus (2×1012vp) encoding the gene for β-Galactosidase (LacZ ) [AAV2 / LacZ]. Immunohistochemical staining for β-Galactosidase in heart indicated high efficiency transduction of the majority of cardiomyocytes in treated vs. control, non-treated animals (see FIG. 6). The Ad / LacZ was made as described in Hoshijima, M., et al. Nat. Med. 8:864-871 (2002).
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com