Use of RyR2 protein or RyR2 recombinant protein in preparation of anti-heart failure drug
A recombinant protein, heart failure technology, applied in drug combinations, peptide/protein components, recombinant DNA technology, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
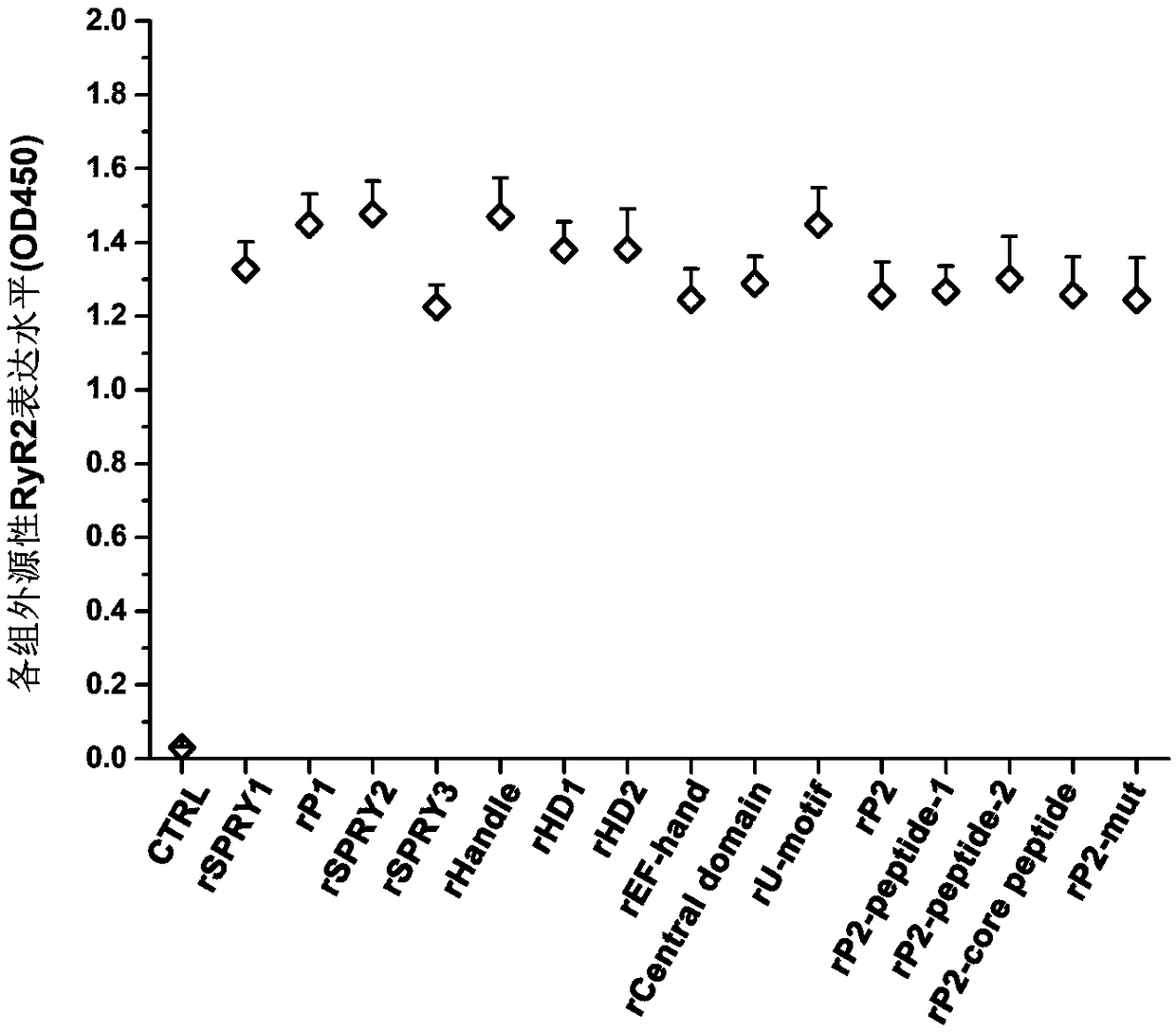
[0058] Example 1. Effects of Myocardium-Specific Exogenous Expression of RyR2 Protein on Cardiac Function in Normal Small Animal Models
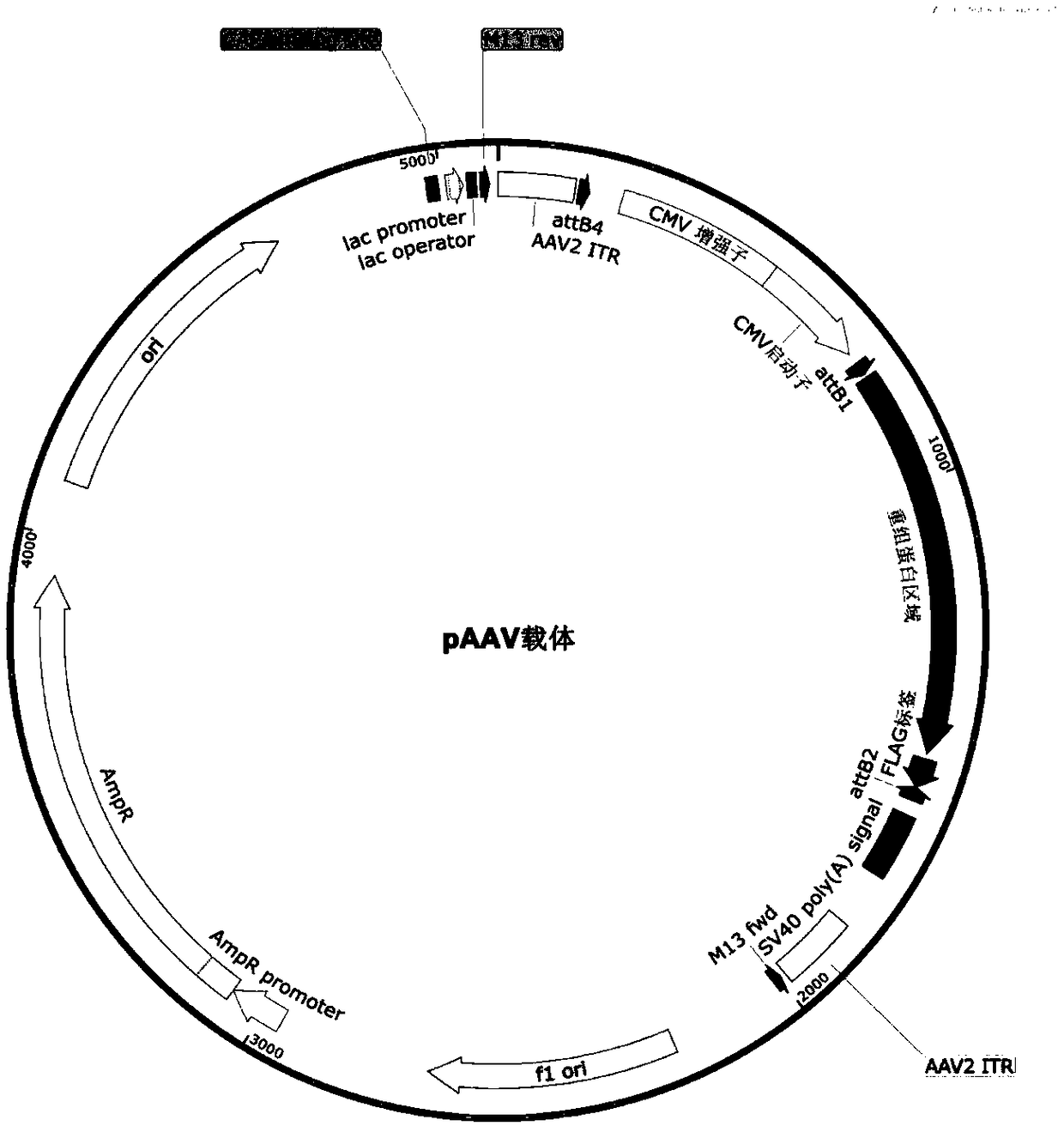
[0059] In order to detect the enhancement effect of AAV9-mediated RyR2cDNA myocardial gene transfer on cardiac function, 6-month-old normal C57B / 6 mice were divided into treatment group and control group. The intracellular delivery of all RyR2 recombinant proteins uses pAAV vectors, and FLAG tags are added to the C-terminus for easy detection. The viral vector construction diagram is as follows figure 1 . The polynucleotide encoding the following recombinant protein is the recombinant protein region of the carrier as shown in the figure, and the ATG start codon is added at the 5' end if necessary. The specific groups are as follows:
[0060] The CTRL control group is the empty virus group; the rSPRY1 group is the recombinant SPRY domain protein group, namely figure 1 The polynucleotide of the recombinant protein region of the vector show...
Embodiment 2
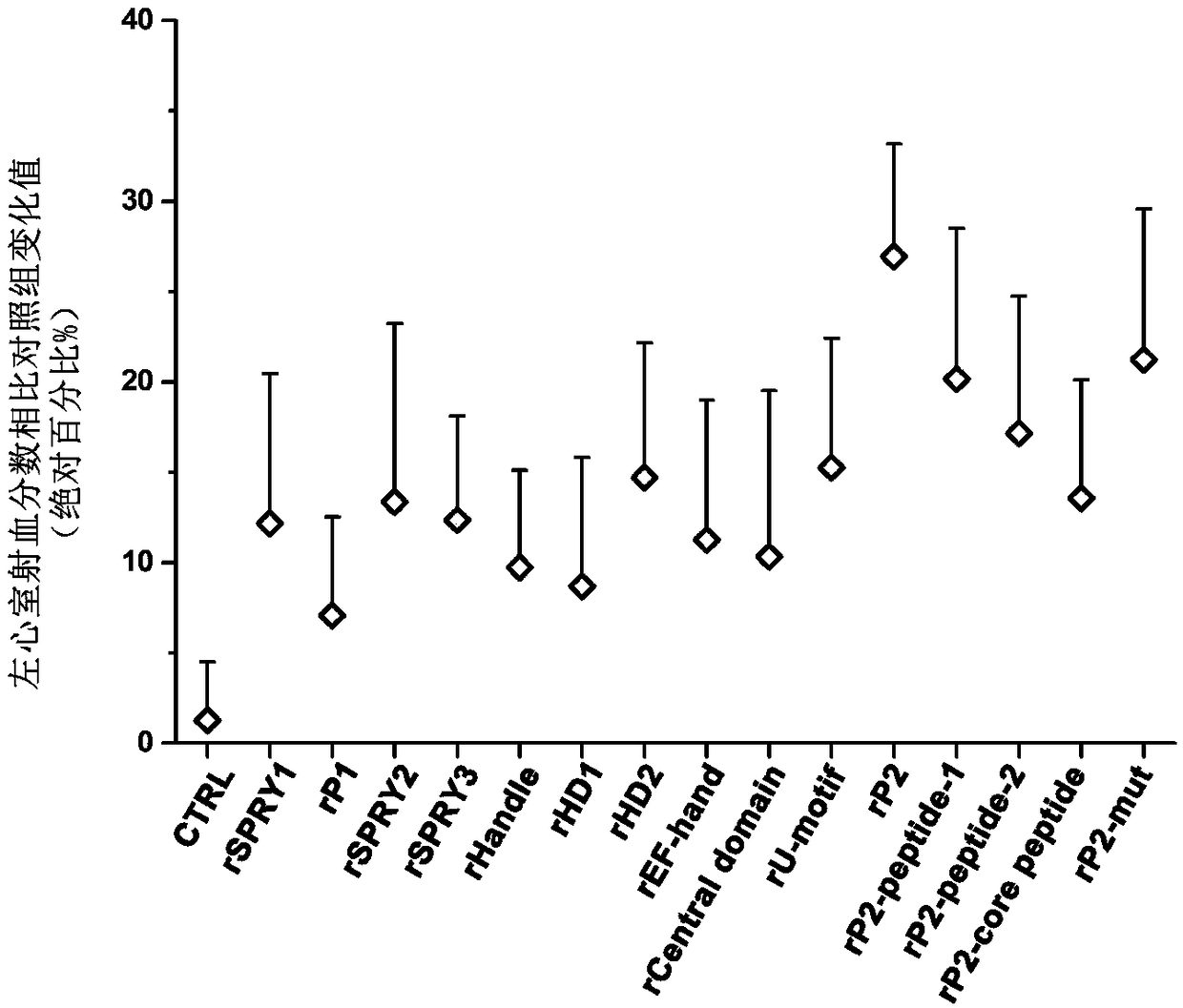
[0065]Example 2. Effects of Myocardium-Specific Exogenous Expression of RyR2 Protein on Cardiac Function in Small Animal Disease Models
[0066] Further, to evaluate the effect of AAV9-mediated RyR2cDNA myocardial gene delivery on cardiac function enhancement in disease models, the steps are as follows:
[0067] First, a 6-month-old C57B / 6 mouse myocardial infarction model was established. The model was established by temporarily occluding the left anterior coronary artery, referring to the non-patent literature Brinks et al.Circ Res (2010) 107:1140-1149; then, the The mice were divided into a treatment group and a control group. All RyR2 protein C-terminals were tagged with FLAG to facilitate detection. The specific construction method was the same as in Example 1. The virus cardiomyocyte infection technique, the construction of the virus vector and the method of using the myocardial specific promoter are the same as before, and the injection dose of the virus particles is 1×...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com