Controlled release delivery system for nasal applications
a delivery system and release technology, applied in the direction of biocide, plant/algae/fungi/lichens, drug compositions, etc., can solve the problems of limited use in clinical practice, limited possibilities for formulating nasal application forms, short time available for absorption, etc., and achieve favorable serum level profile and high bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example
[0060] Typical Formulation
[0061] The formulation shown below was selected considering the serum level of the active ingredient achieved but it also exhibits a skin care property which is important for long term applications.
TABLE 1Most preferred formulationCompoundAmount per containerDelivery per sprayTestosterone 2% ≈2.8 mgAerosil ® 200 3% ≈4.2 mgLabrafil ® M 1944 CS 4% ≈5.6 mgCastor oil, refined grade91%≈127.4 mg
[0062] Typical Serum Level
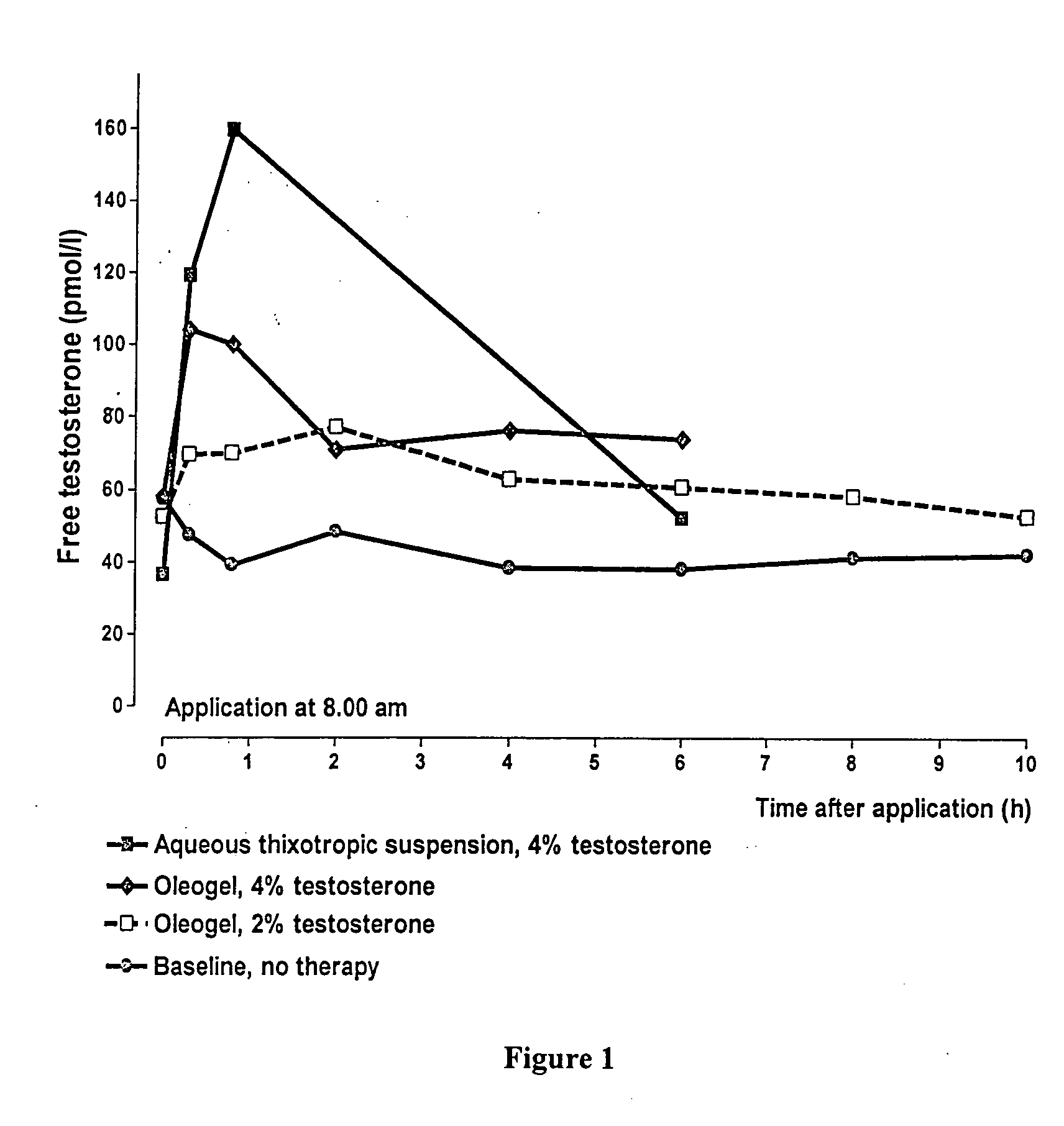
[0063] Comparing different formulations (see FIG. 1) containing testosterone it is obvious that Cmax is clearly decreased in the special oily formulation of this invention, which is desirable in view of toxicological considerations. Further the level of unbound testosterone is very constant over at least 10 hours mimicking the physiologic daily rhythm of testosterone release.
[0064] The dotted line shows the serum level after application of 1 spray per nostril once of the most preferred formulation (see Table 1).
[0065] It can be concluded tha...
PUM
| Property | Measurement | Unit |
|---|---|---|
| lipophilic | aaaaa | aaaaa |
| surface tension | aaaaa | aaaaa |
| weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com