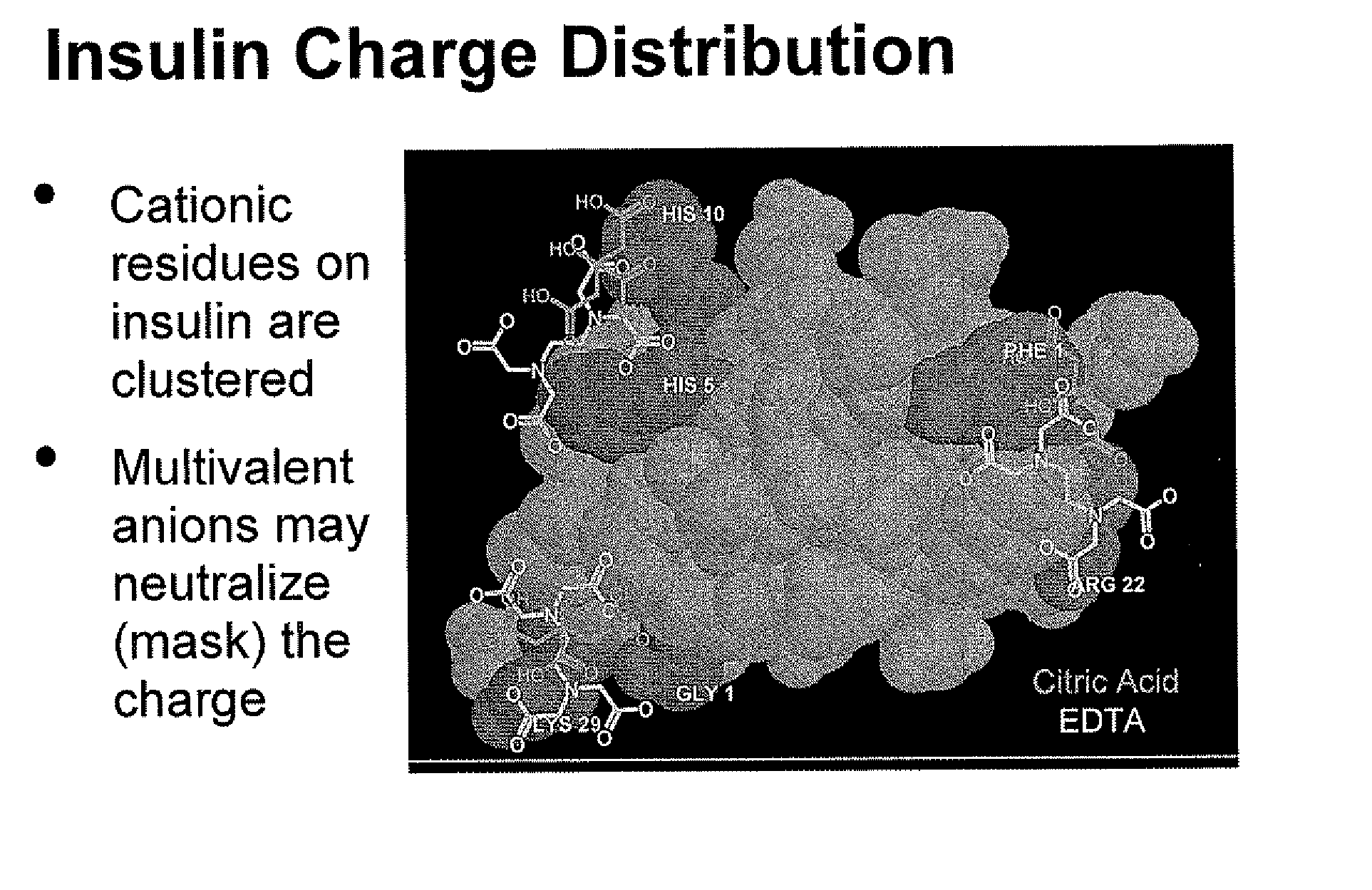
Rapid Acting Injectable Insulin Compositions
a technology of insulin composition and injection, which is applied in the direction of peptide/protein ingredients, extracellular fluid disorder, metabolic disorder, etc., can solve the problems of interfering with the ability of proteins, abnormally high blood glucose level of patients with diabetes, and inadequate insulin levels, etc., to achieve rapid onset of action, improve stability, and rapid absorbed into the blood stream
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
In Vitro Comparison of Uptake and Transport of Insulin using Epithelial Cell Transwell Assay as a Function of Dissolution Agent
[0058] Materials and Methods
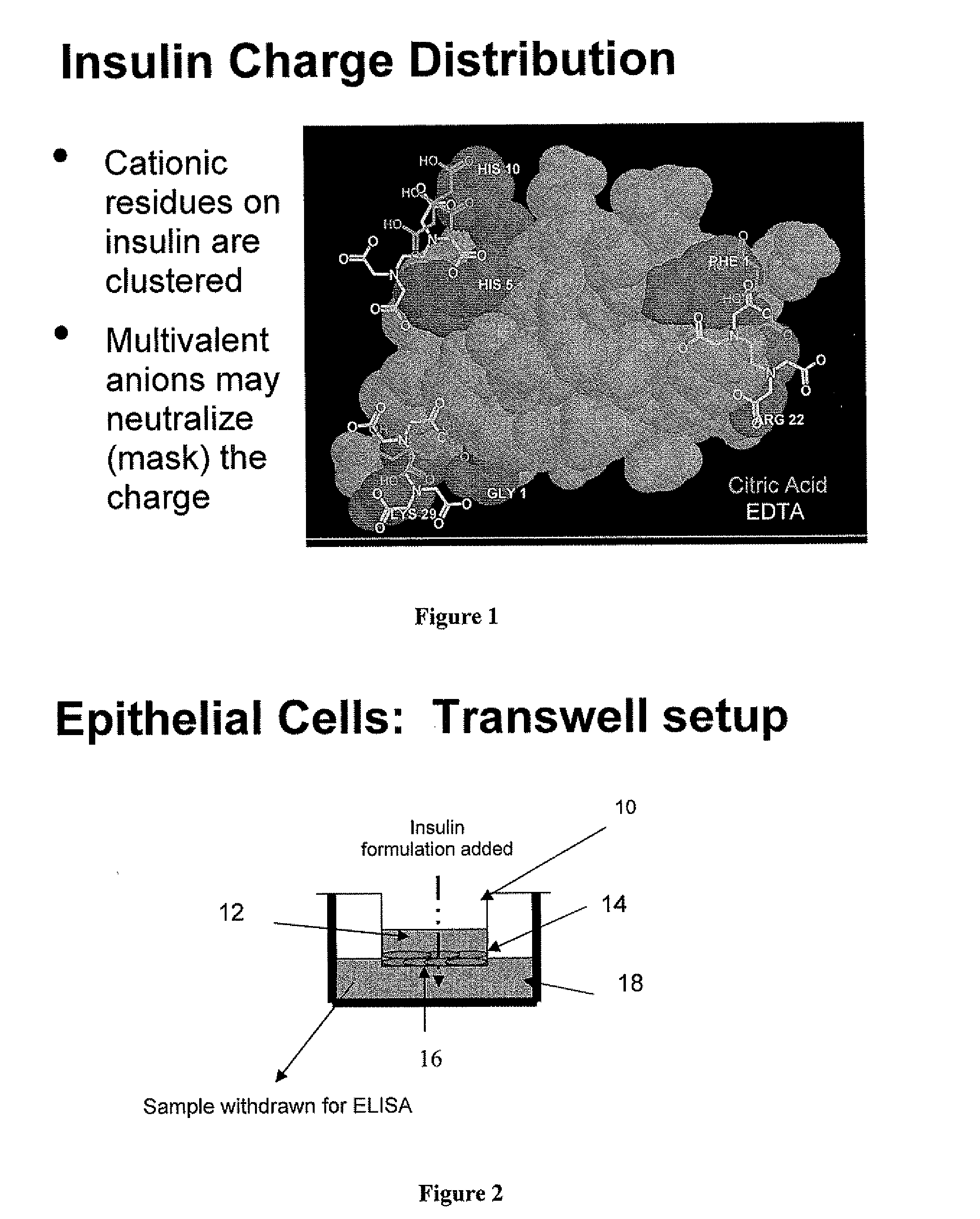
[0059] Oral epithelial cells were grown on transwell inserts for two weeks until multiple (4-5 layer) cell layers had formed, as shown in FIG. 2. The transport studies were conducted by adding the appropriate solutions to the donor well and removing samples from the receiver well after 10 minutes. Solutions consisted of water, + / −EDTA (0.45 mg / ml), NaCl (0.85% w / v), 1 mg / ml insulin and a sufficient amount of acid to maintain the pH at 3.8. Insulin amounts in the receiver wells were assayed using ELISA.
[0060] Results
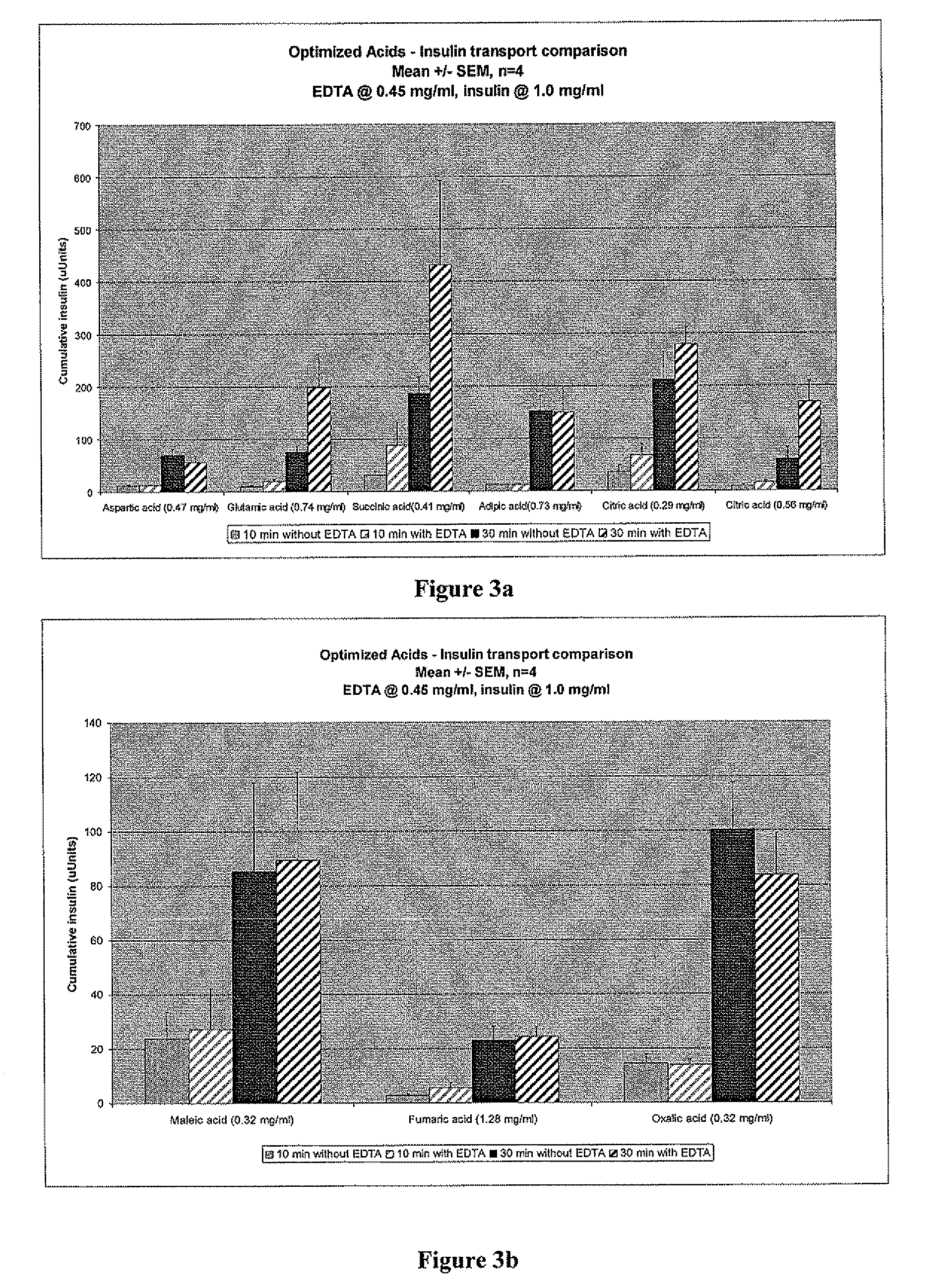
[0061] The results shown in FIGS. 3a and 3b demonstrate that some acids are more effective at enhancing uptake and transport of insulin through epithelial cells. These can be readily tested and compared to the results obtained using HCl, thereby providing a standard against which any acid can be tested and determined...
example 2
In Vitro Comparison of Uptake and Transport of Insulin Using Epithelial Cell Transwell Assay as a Function of Concentration of Dissolution Agent
[0064] Materials and Methods
[0065] The materials and methods of Example 1 were used with different concentrations of reagents. In the study, equimolar concentrations of acid and chelator were added. Solutions consisted of water, + / −EDTA (0.56 mg / mn), NaCl (0.85% w / v), 1 mg / mL insulin and an acid: Aspartic acid (0.20 mg / mL), Glutamic acid (0.22 mg / mL) or citric acid (0.20 mg / ml). Citric acid was tested at a higher concentration of 1.8 mg / mL with and without chelator. This data is shown at two time periods, 10 and 30 minutes, post dosing of cell donor chambers.
[0066] Results
[0067] The results obtained with Aspartic acid (0.20 mg / mL), Glutamic acid (0.22 mg / mL) or citric acid (0.29 mg / ml) are shown in FIG. 4a. In this case, there was no significant difference seen with the addition of the chelator.
[0068] In contrast, the study using a high...
example 3
In Vitro Comparison of Uptake and Transport of Insulin Using Epithelial Cell Transwell Assay as a Function of Chelator
[0069] Materials and Methods
[0070] Oral epithelial cells were grown on transwell inserts for two weeks until multiple (4-5 layer) cell layers had formed. The transport studies were conducted by adding the appropriate solutions to the donor well and removing samples from the receiver well after 10, 20 and 30 minutes.
[0071] The solutions were prepared immediately before the transwell experiments in the following way: Citric acid at 1.8 mg / ml was dissolved in 0.85% w / v saline and then one of the following chelators was added to this solution at the concentration shown: EDTA at 1.80 mg / ml, EGTA at 1.84 mg / ml, DMSA at 0.88 mg / ml and TSC at 1.42 mg / ml. Because CDTA was used in its liquid form, citric acid was added directly to the CDTA. In each of these cases, the concentration of chelator was constant at 4.84×10−3 moles.
[0072] Insulin was then added at 1 mg / ml and the...
PUM
| Property | Measurement | Unit |
|---|---|---|
| time periods | aaaaa | aaaaa |
| time periods | aaaaa | aaaaa |
| concentrations | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com