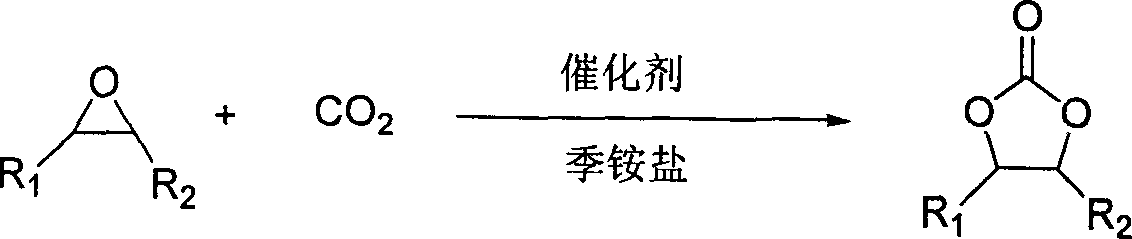
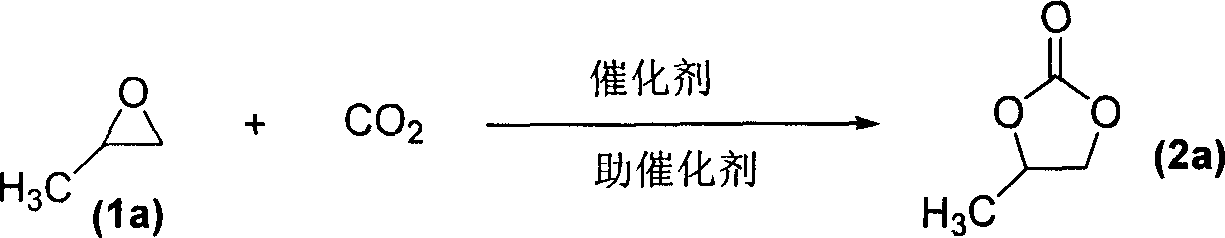
Method for synthesizing cyclic carbonate from carbon dioxide and epoxy compound through reaction of cycloaddition
A technology of epoxy compounds and cyclic carbonates, applied in chemical recovery, organic chemistry, etc., can solve the problems of difficult separation of products and catalysts, high cost of catalysts, harsh conditions, etc., and achieve low cost, simple catalyst system and excellent reaction conditions mild effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019]
[0020] In a 70 ml autoclave, 0.05 mmol of chitosan (CS)-loaded zinc chloride (based on metal content), 0.3 mmol of cocatalyst BMImBr, and finally 10 ml of propylene oxide (1a) were added in sequence. The reaction kettle was sealed, and the temperature was controlled by a temperature controller to slowly rise to 110° C. to react for 1 hour. During the reaction, the reaction pressure was kept constant. After the reaction, the reactor is cooled to room temperature, and carbon dioxide is slowly released, and the liquid obtained from the reaction is filtered to separate the catalyst for recycling. The filtrate was distilled under reduced pressure to obtain the corresponding propylene carbonate (2a). The selectivity is 99%, and the conversion frequency (TOF) is 2717h -1 .
Embodiment 2
[0022] With embodiment 1, catalyst used is CS-FeCl 3 , to obtain propylene carbonate (2a). The selectivity is 99%, and the conversion frequency (TOF) is 2000h -1 .
Embodiment 3
[0024] With embodiment 1, catalyst used is CS-AlCl 3 , to obtain propylene carbonate (2a). The selectivity is 99%, and the conversion frequency (TOF) is 1500h -1 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com