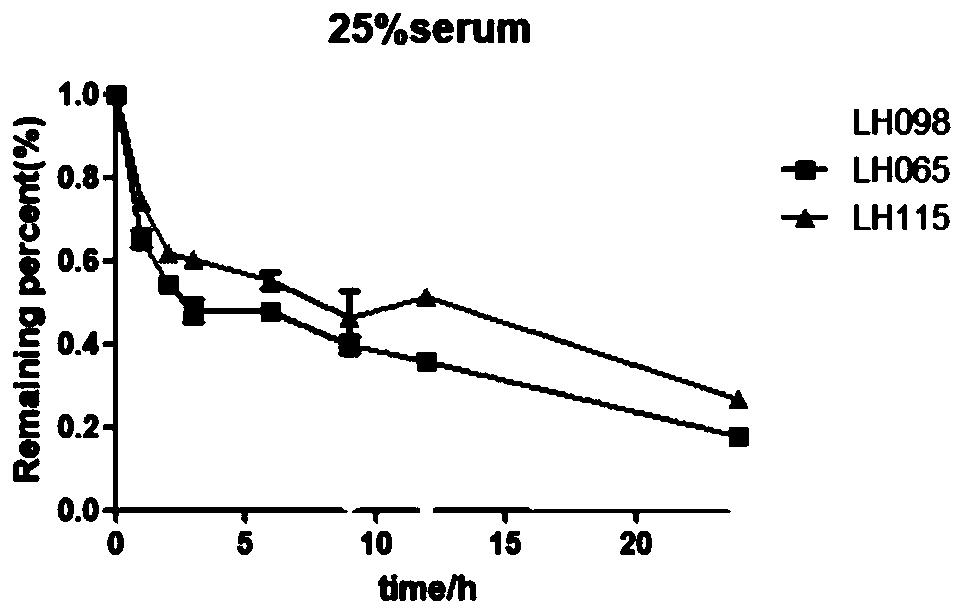
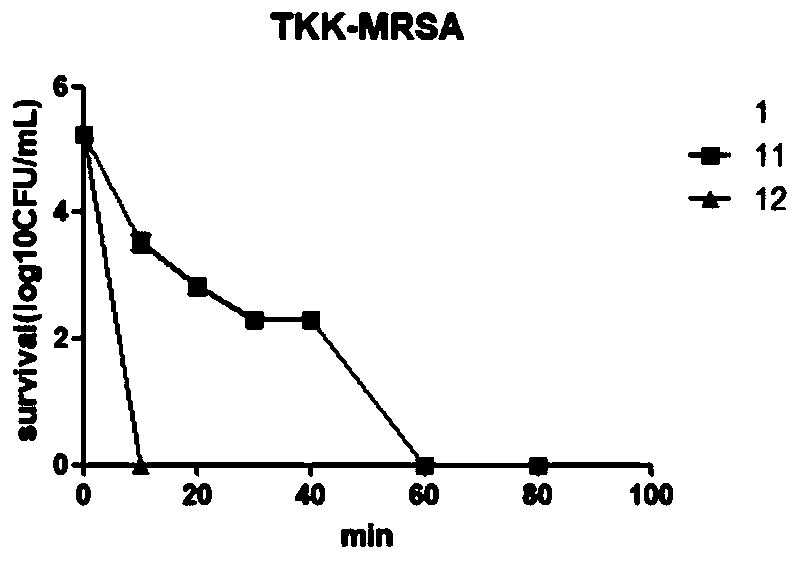
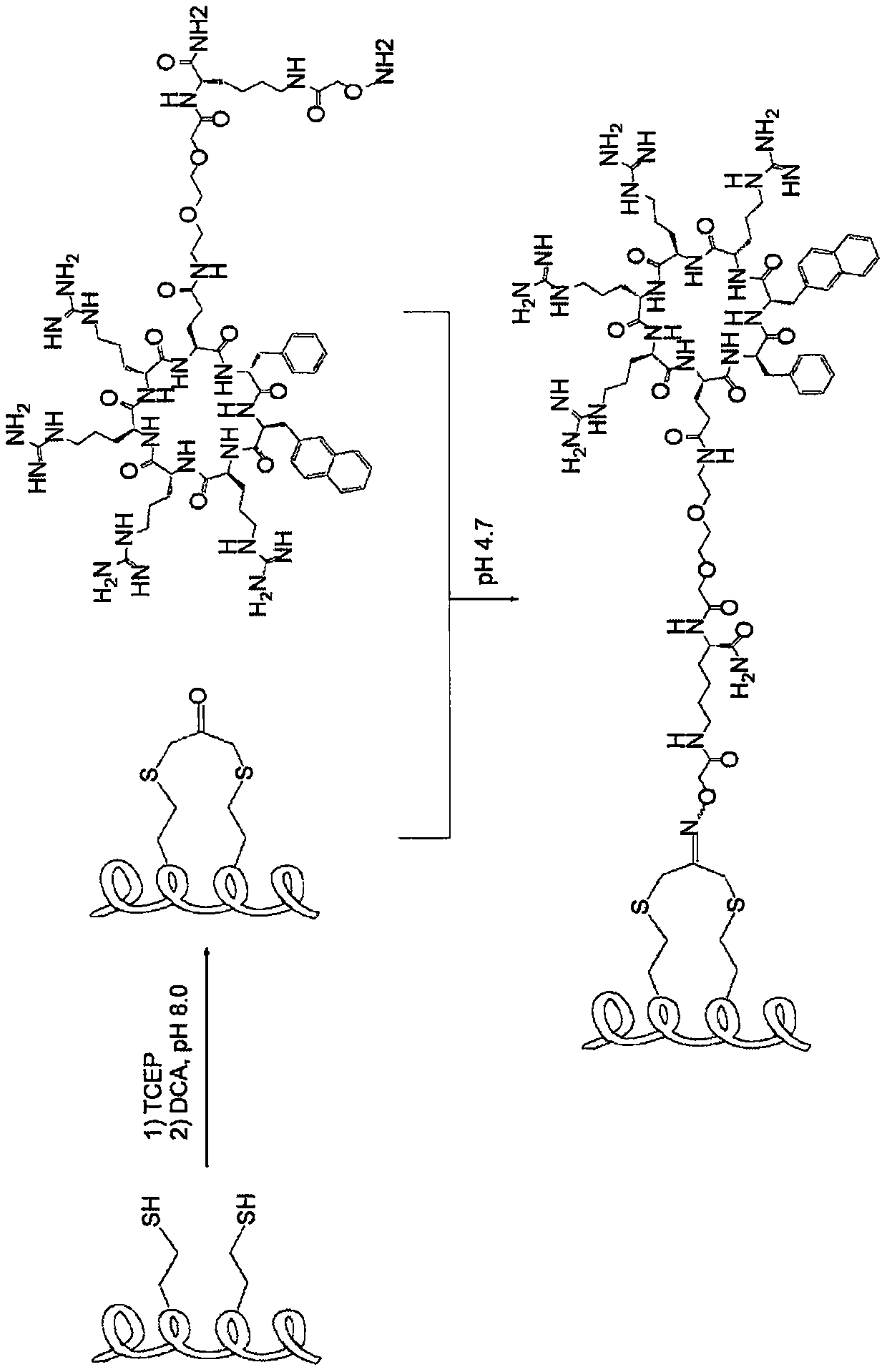
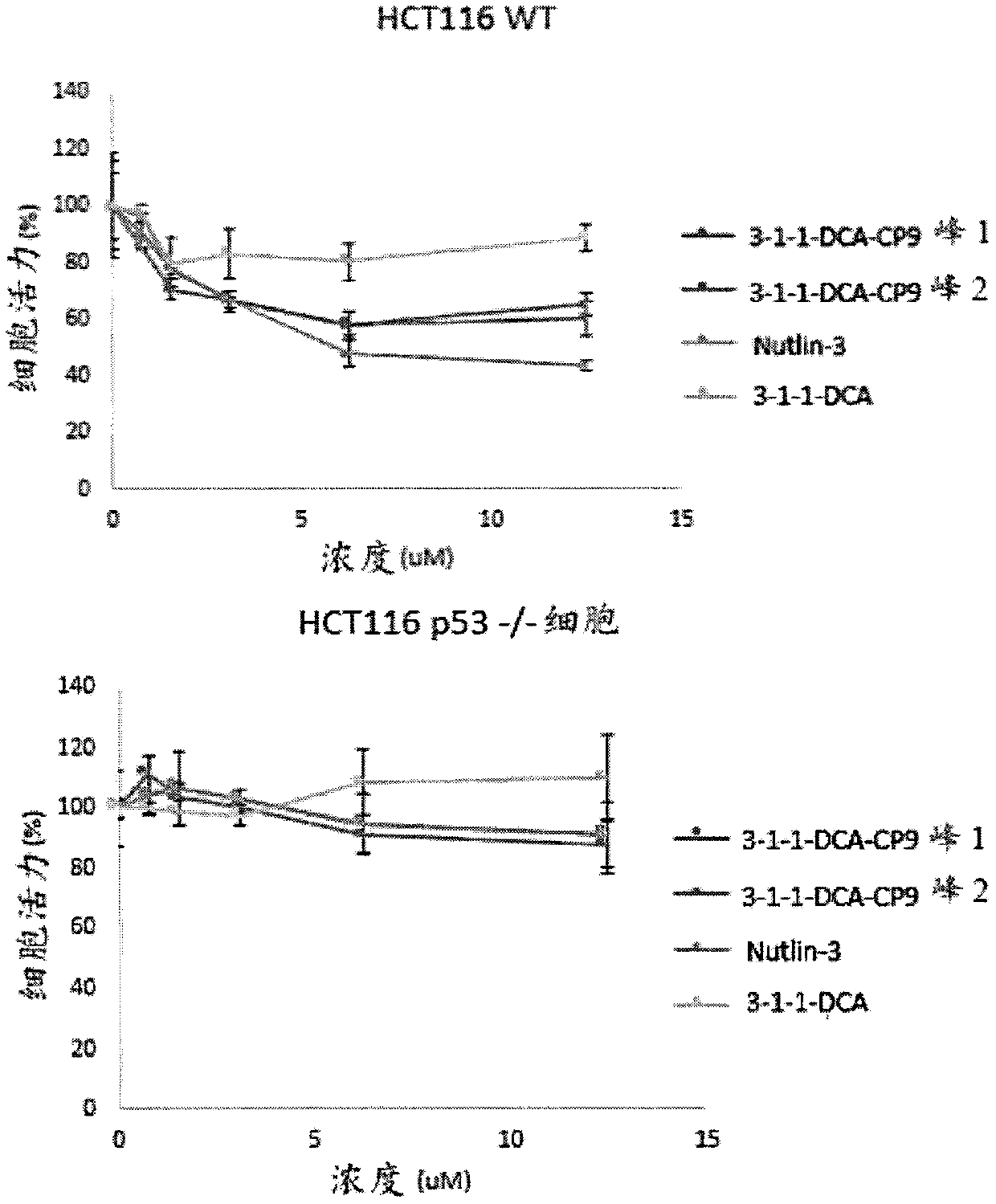
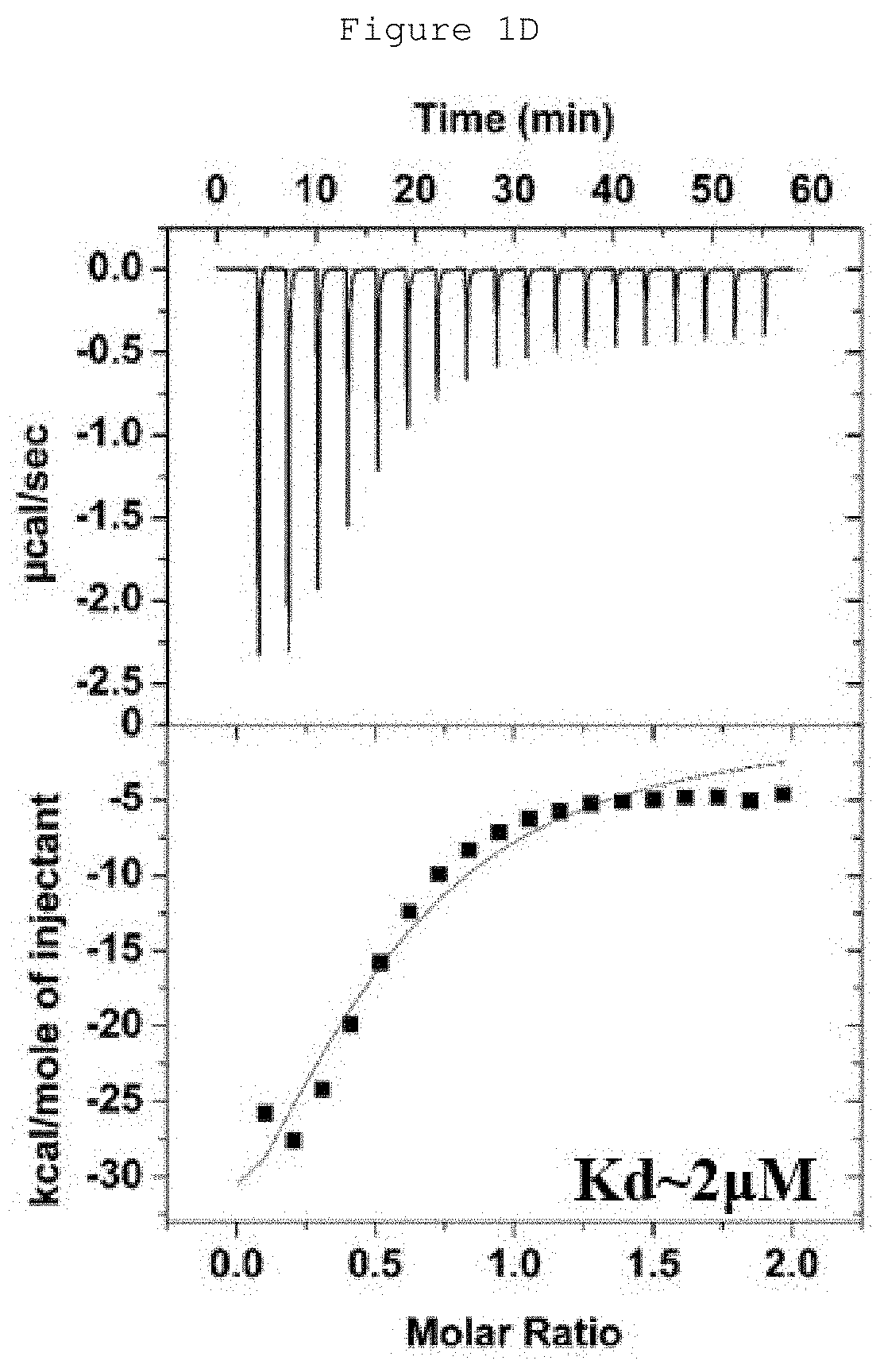
Patents
Literature
46 results about "Stapled peptide" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
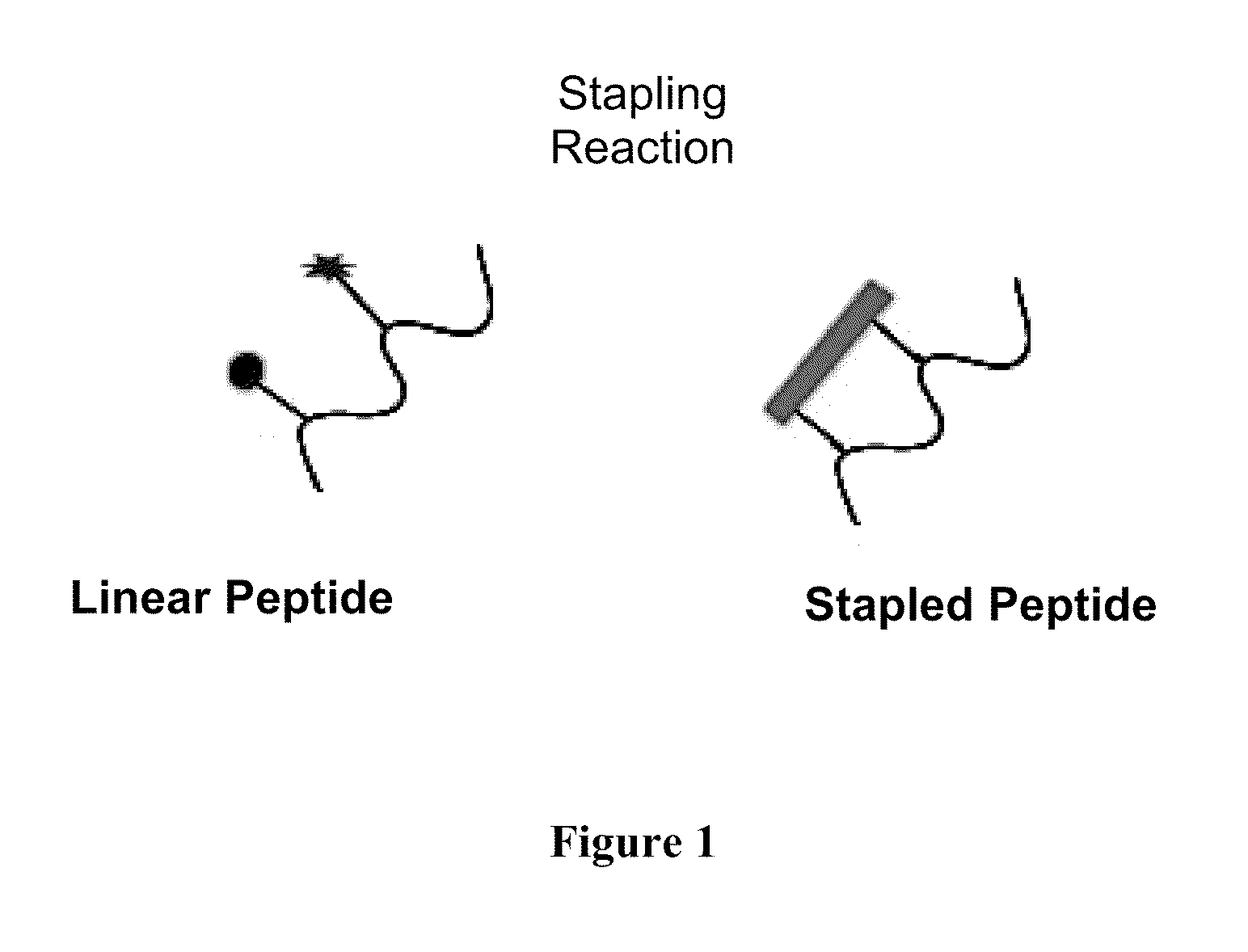
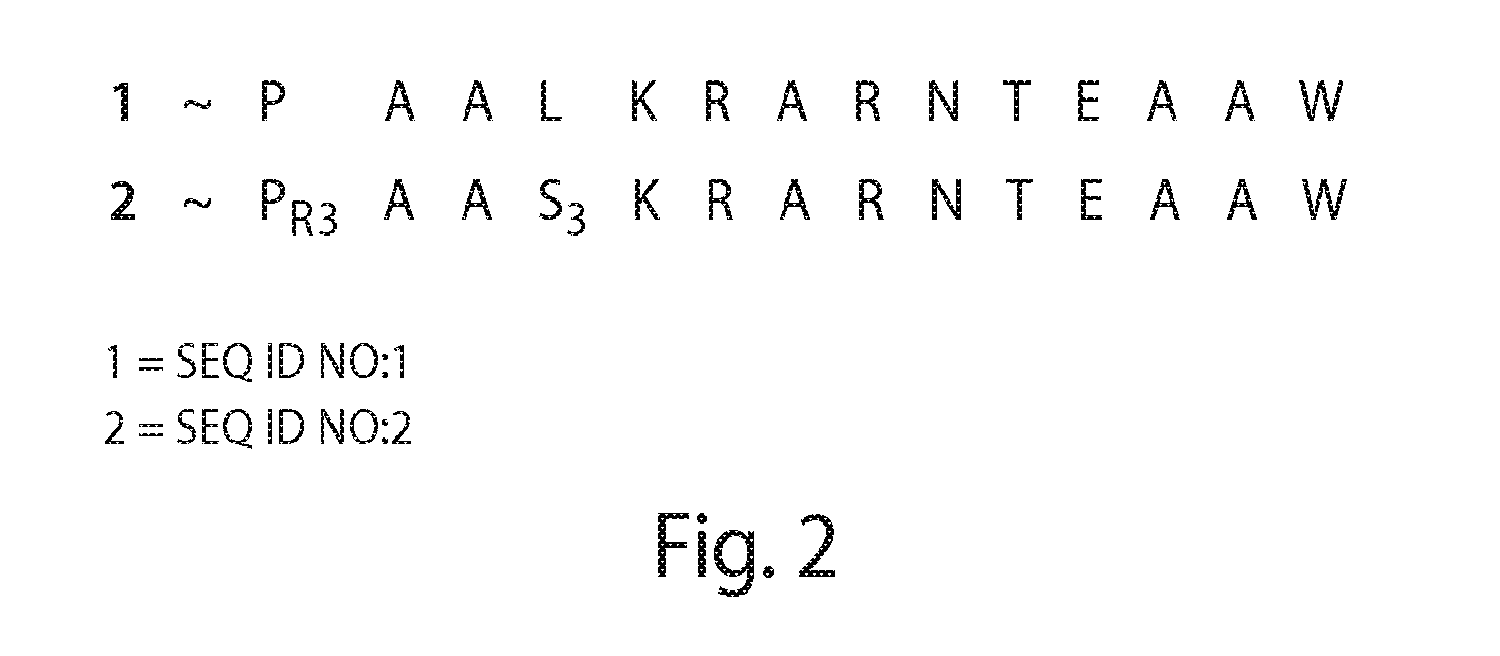
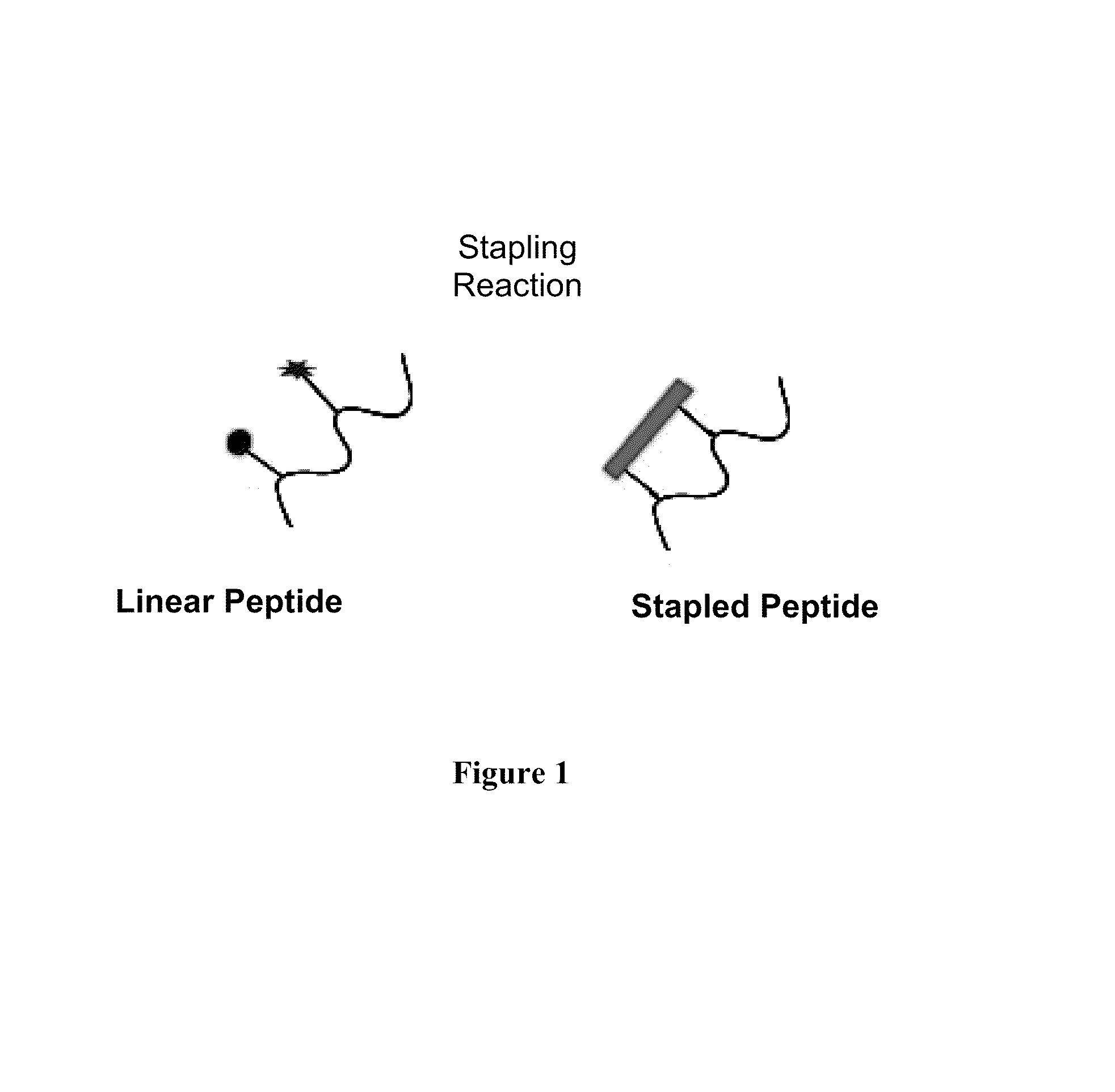
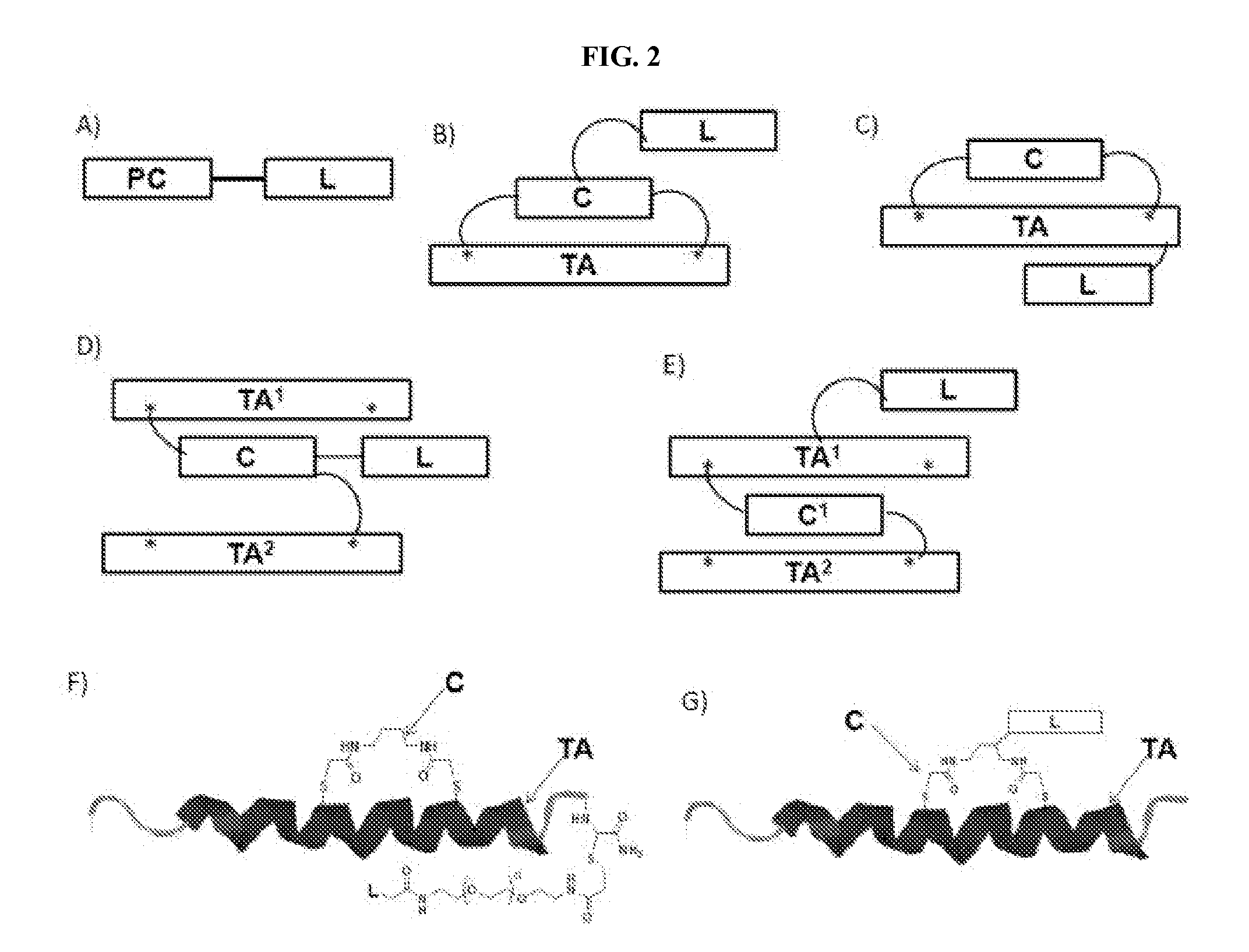
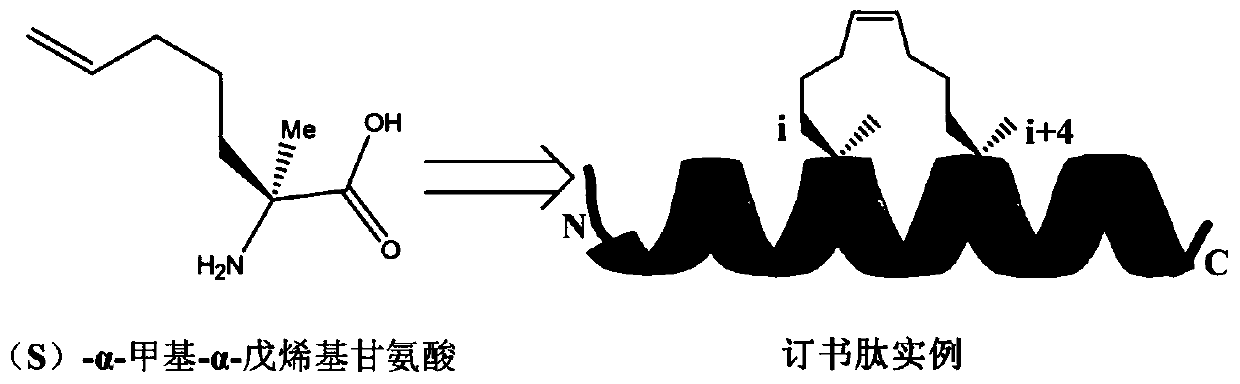
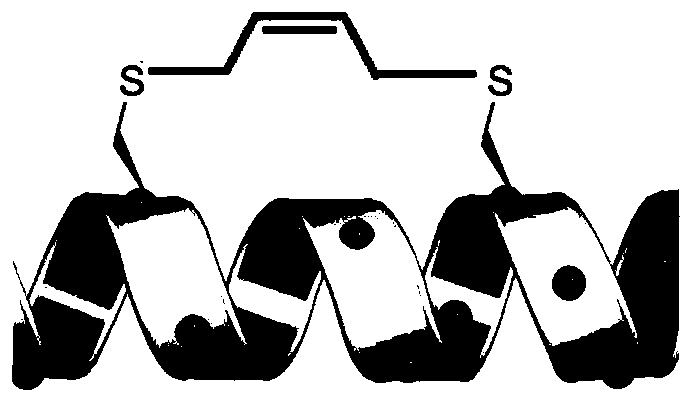
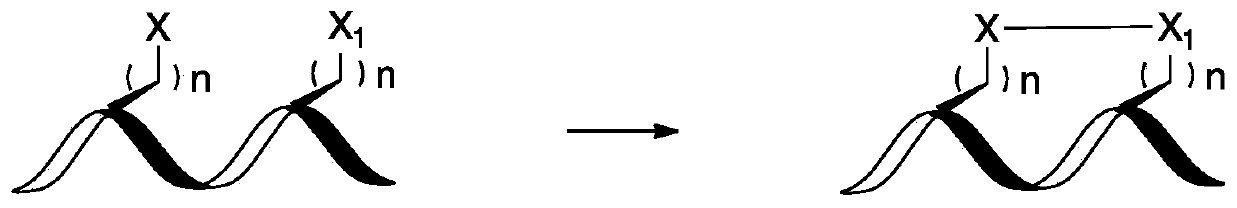
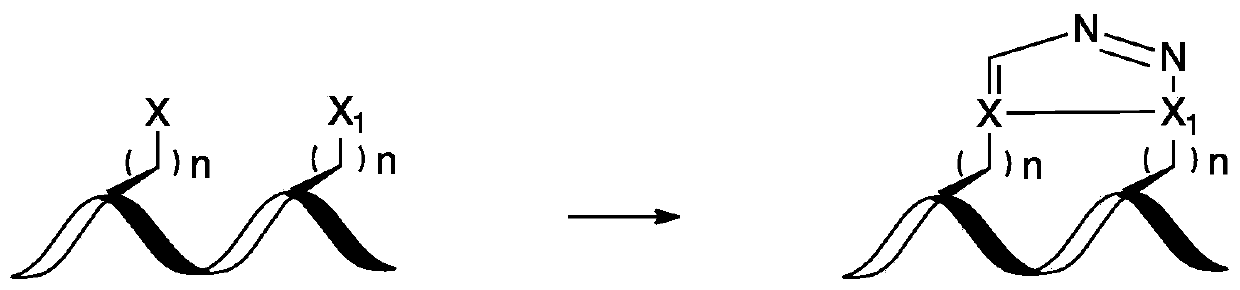
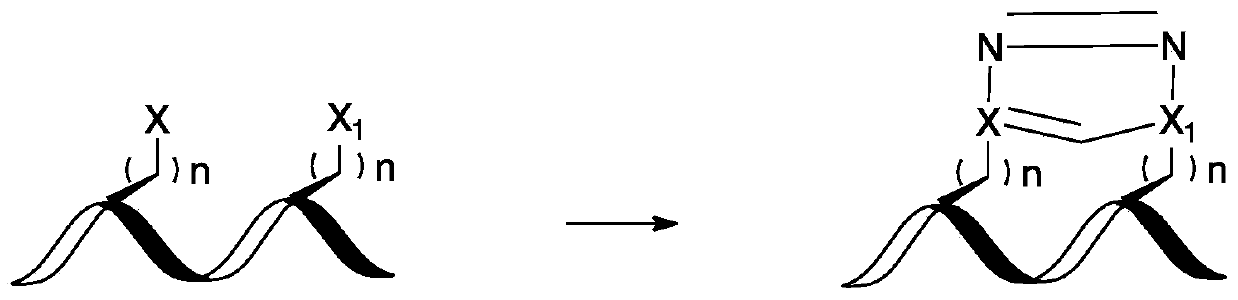
A stapled peptide is a peptide that has a synthetic brace ("staple").
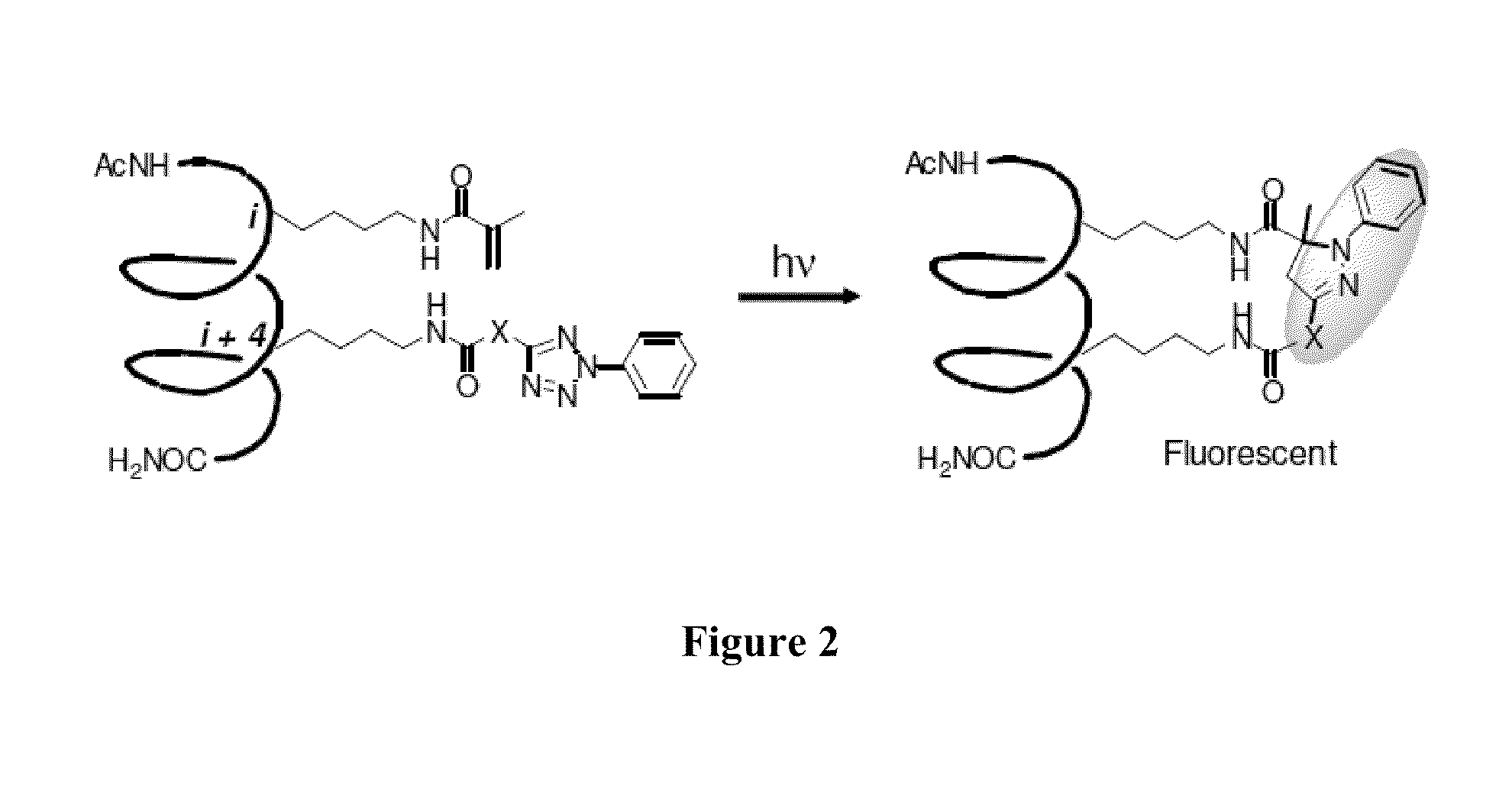
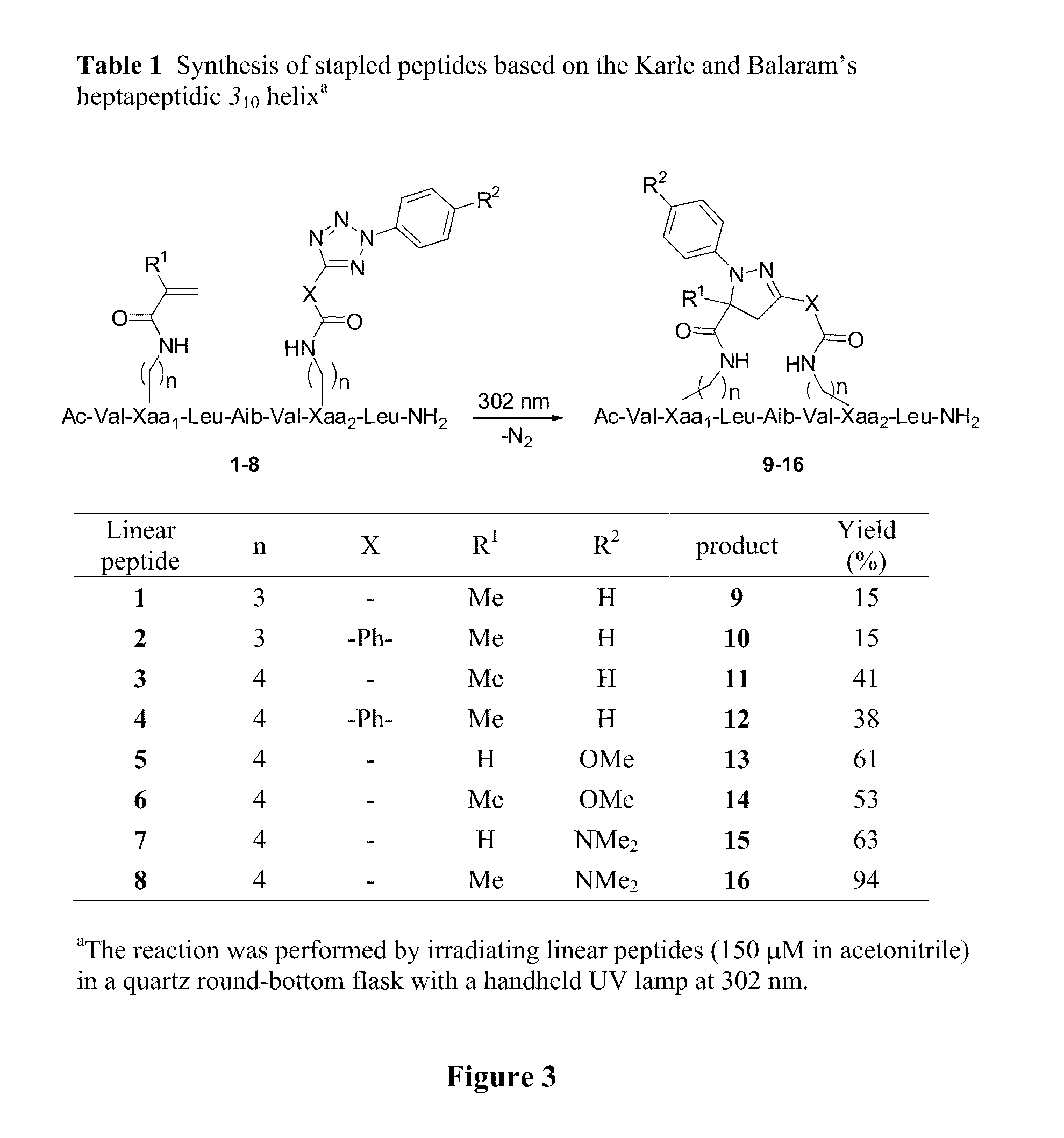
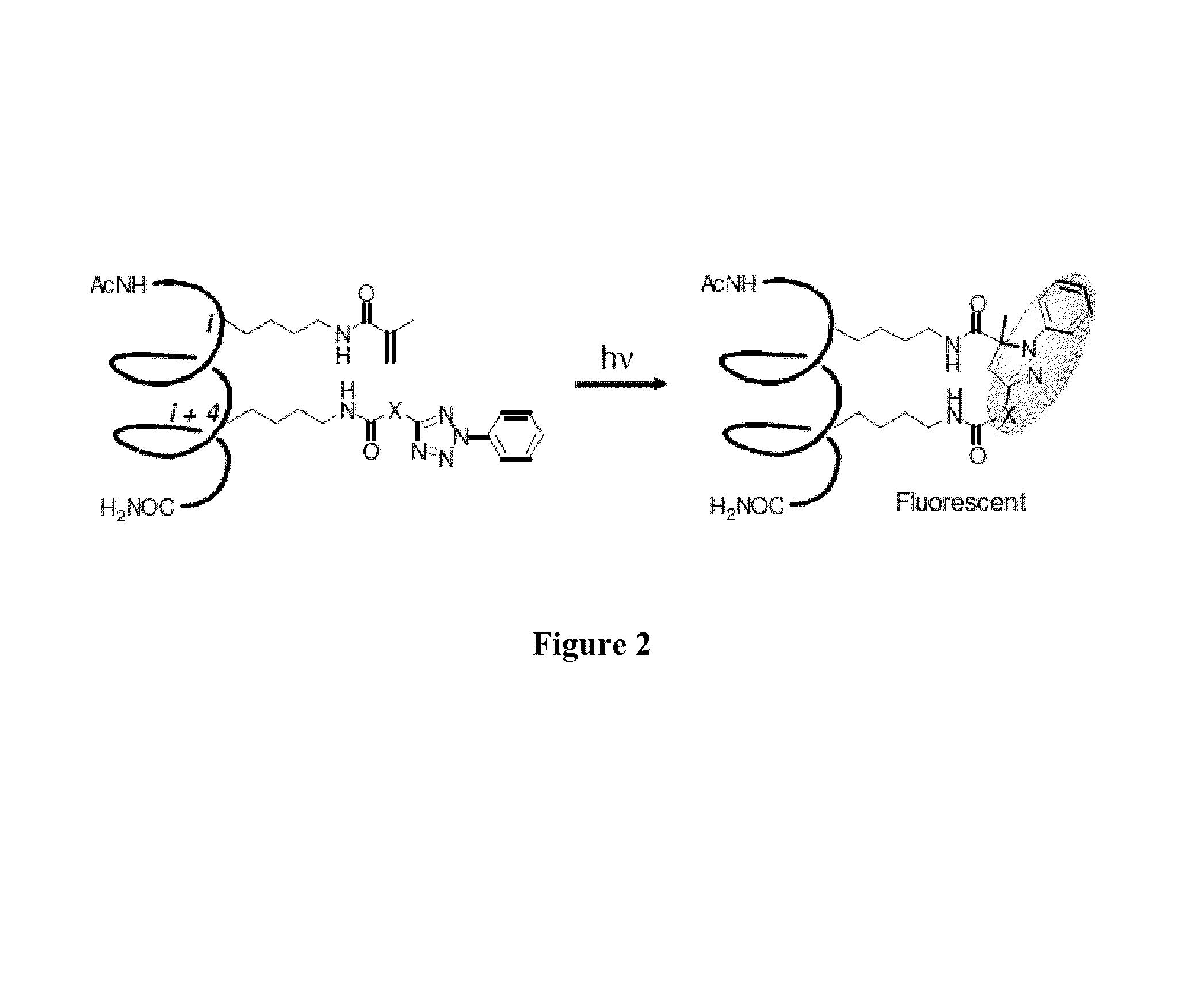
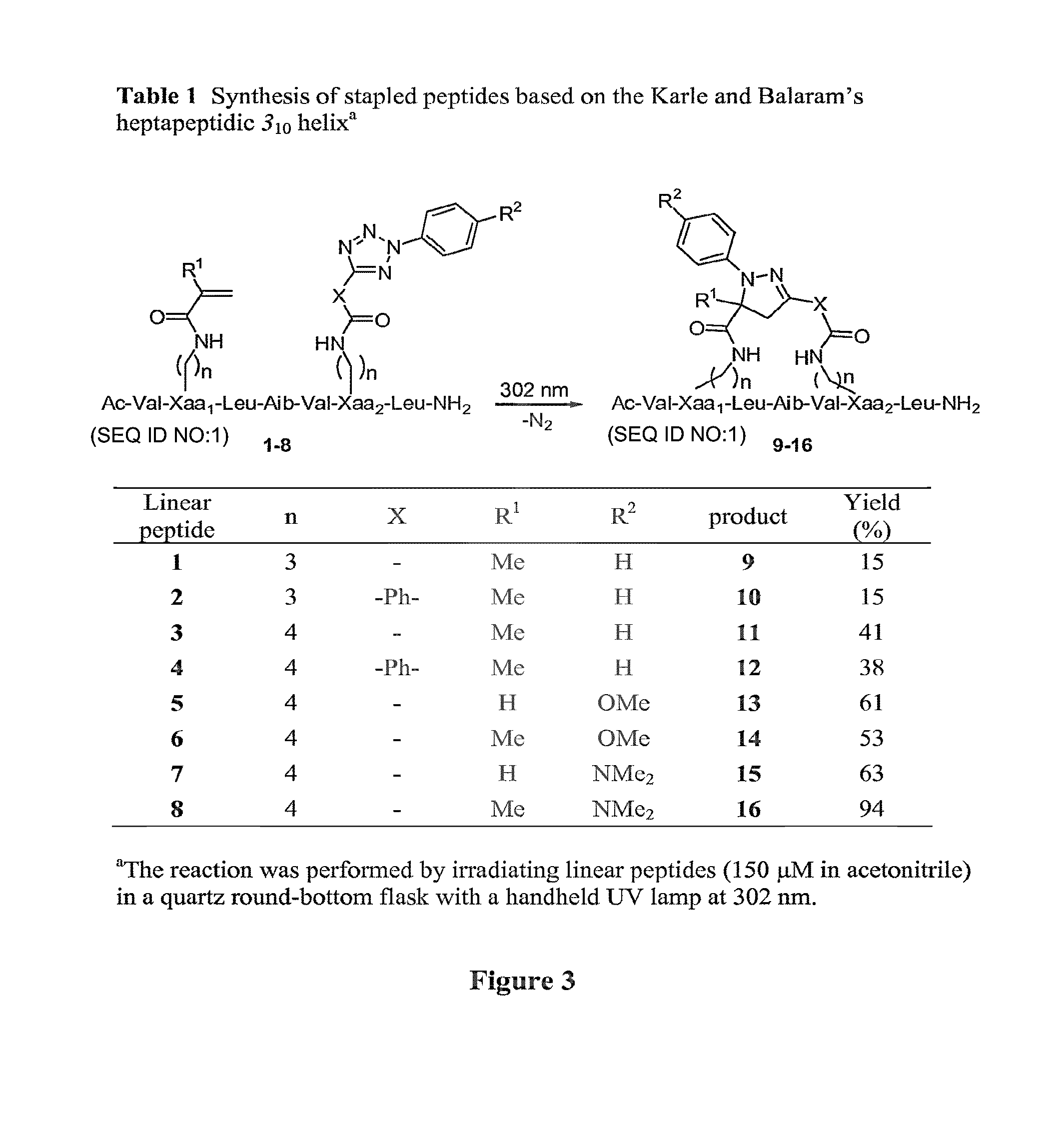
Stapled Peptides and Method of Synthesis
ActiveUS20100093086A1Reasonable reaction yieldSimple procedurePeptide/protein ingredientsPeptide sourcesCycloadditionStapled peptide
A method for preparing stapled peptides. The stapled peptides, including helical stapled peptides, are prepared according to a photochemically-based method, a [3+2] cycloaddition reaction. The helical stapled peptides exhibit increased helicity, thermal stability and cell permeability.
Owner:THE RES FOUND OF STATE UNIV OF NEW YORK
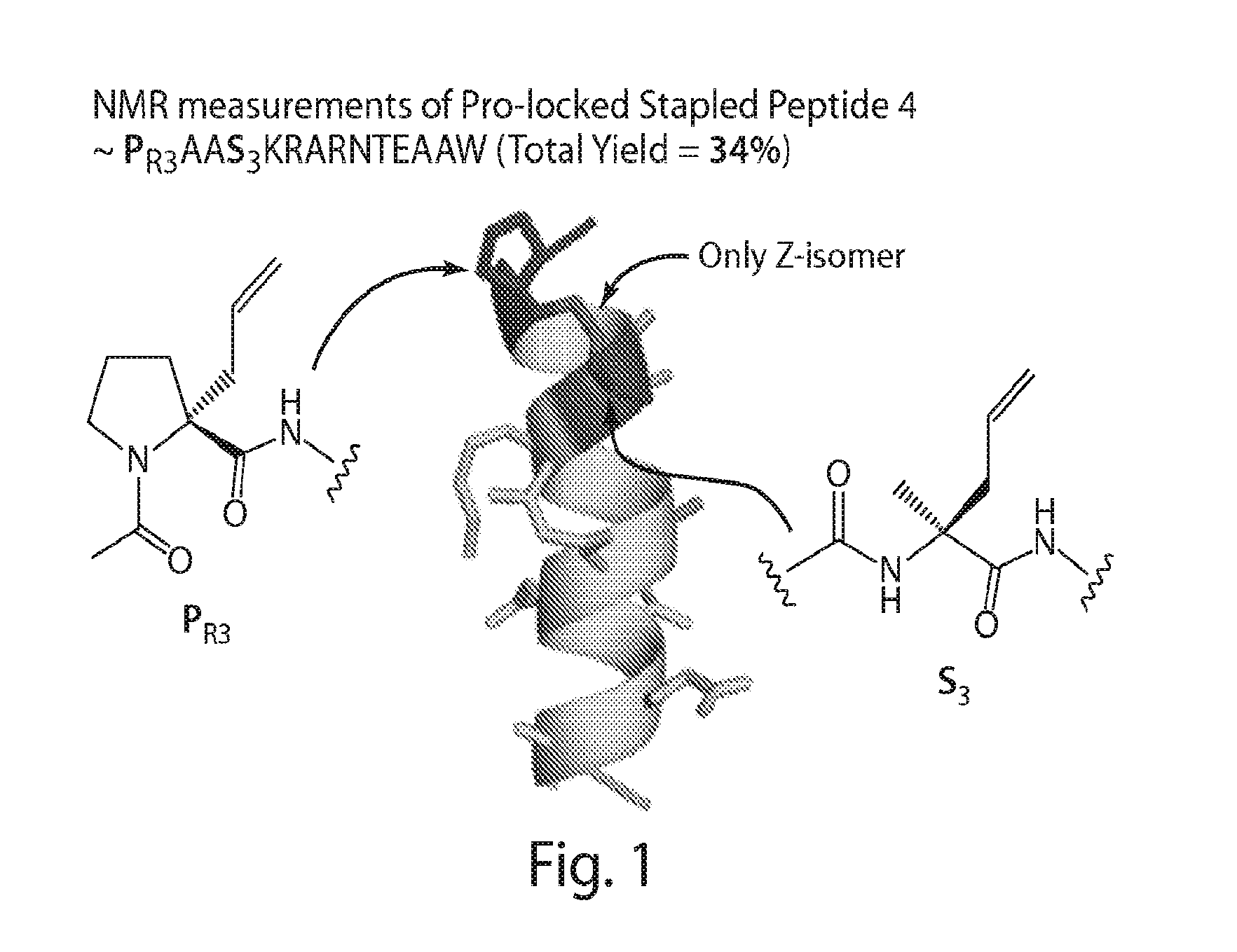
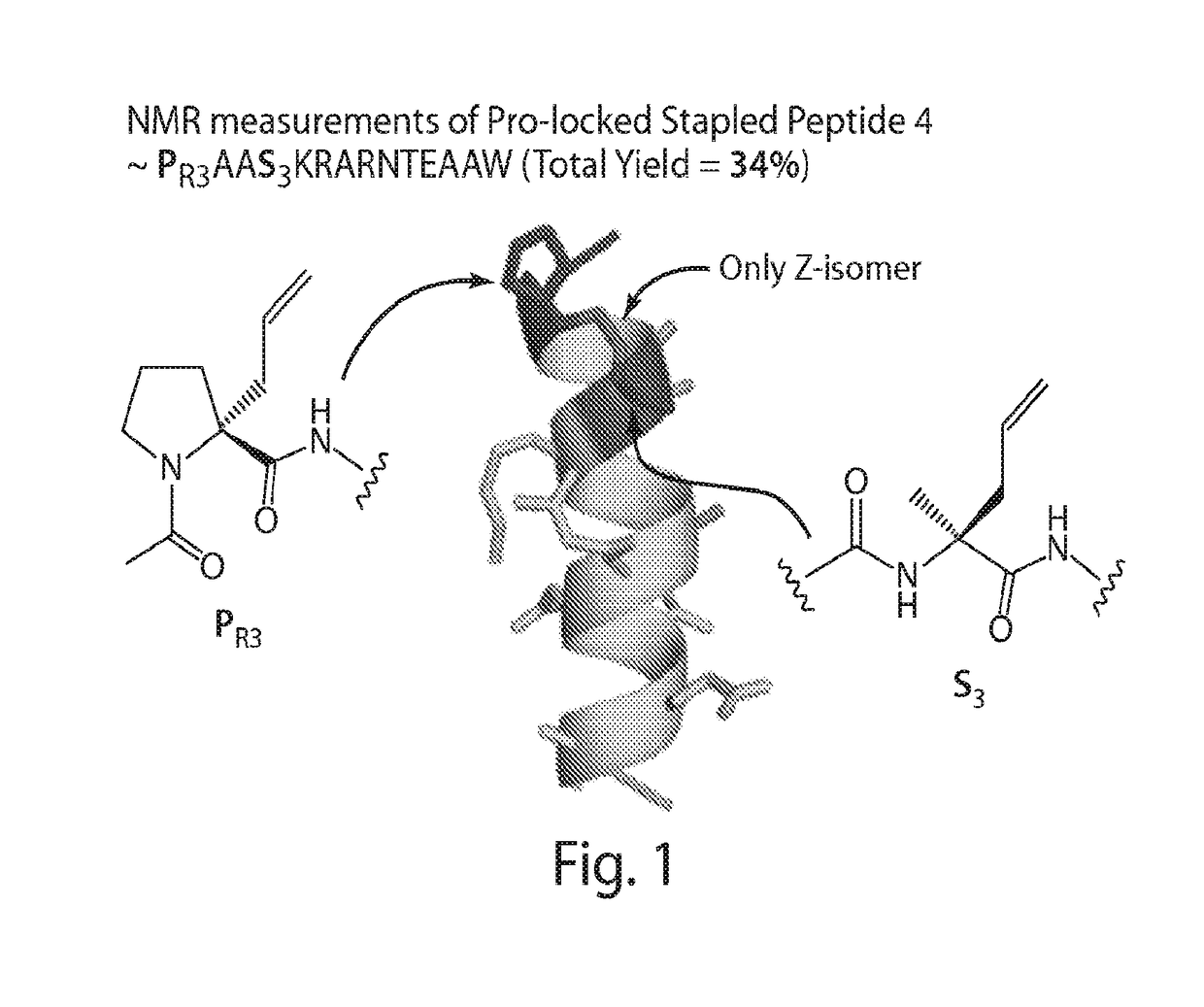
Proline-locked stapled peptides and uses thereof
ActiveUS20150239937A1Oral bioavailabilityImprove stabilitySenses disorderNervous disorderCross-linkSide chain
Owner:PRESIDENT & FELLOWS OF HARVARD COLLEGE
Stapled and stitched polypeptides and uses thereof
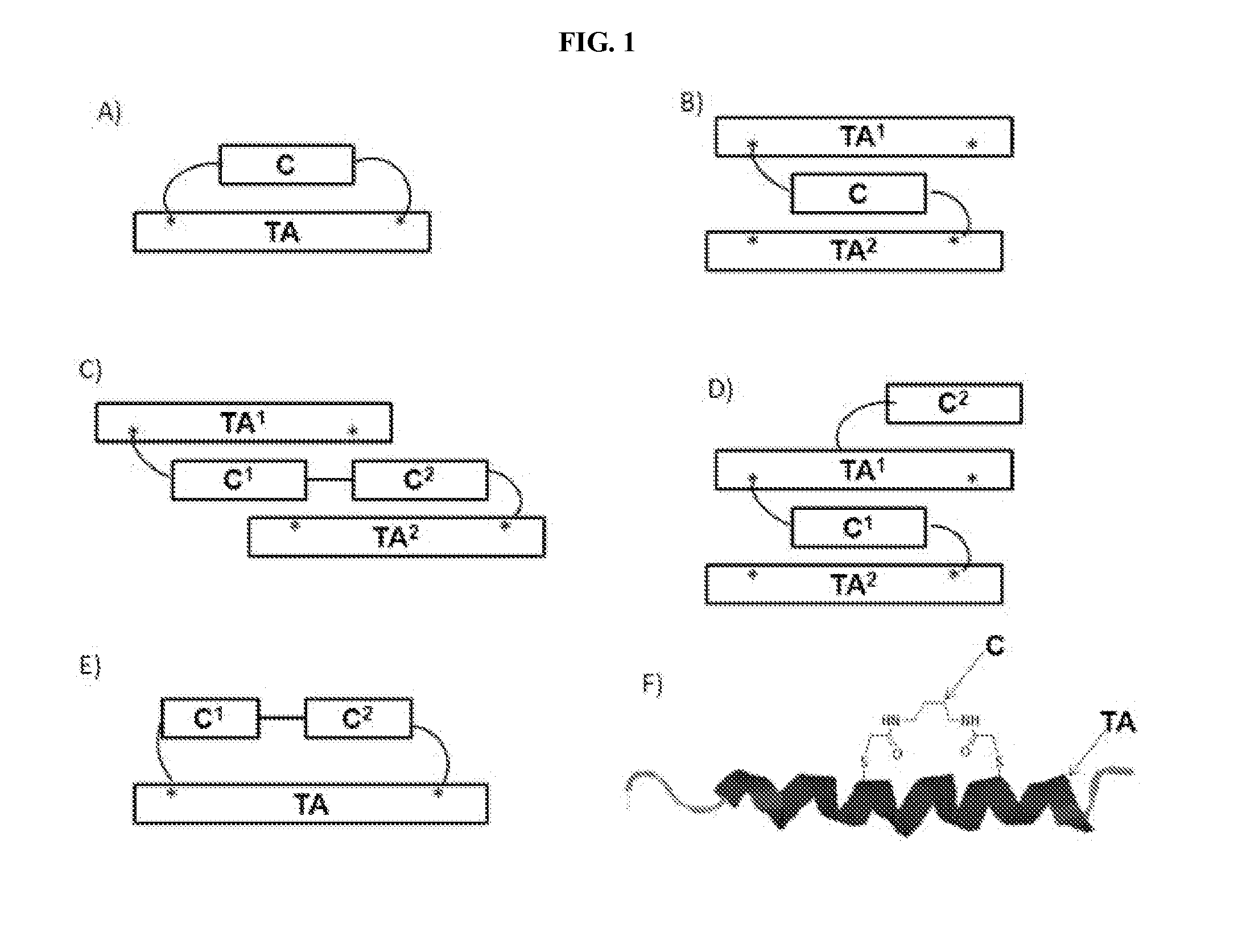
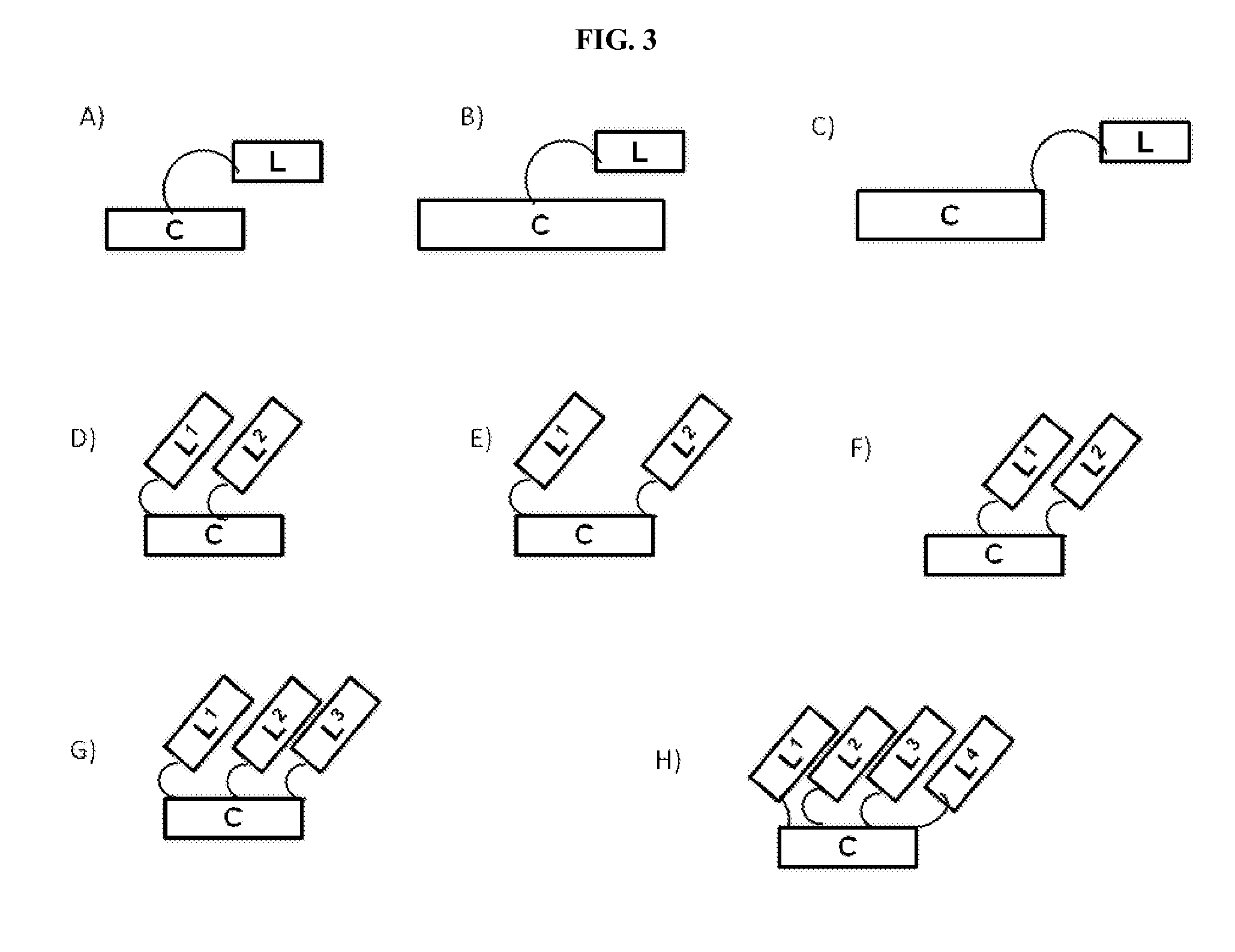
The present invention provides stapled polypeptides of the Formulae (I) and (VI): (I) (VI) and salts thereof; wherein the groups =====; R1a, R1b, R1c, R2a, R3a, R2b, R3b, R4a, R4b, RA, RZ, L1a, L1b, L2, L3, XAA, v, w, p, m, s, n, t, and q are as defined herein. The present invention further provides methods of preparing the inventive stapled polypeptides from unstapled polypeptide precursors. The present invention further provides pharmaceutical compositions comprising a stapled polypeptide of Formula (I) or (VI), and methods of using the stapled peptides. The present invention also provides modifications of the staples post ring closing metathesis.
Owner:PRESIDENT & FELLOWS OF HARVARD COLLEGE
Proline-locked stapled peptides and uses thereof
ActiveUS9617309B2Oral bioavailabilityImprove stabilitySenses disorderNervous disorderCross-linkSide chain
The present invention provides a new type of alpha-helix nucleating cross-link (“staple”) formed by olefin metathesis of a proline derivative with an alkenyl side chain and another amino acid derivative with an alkenyl side chain. The proline derivatives as described herein have been found to be strong nucleators of alpha-helix formation. The invention also provides moieties for shielding the free amide N—H's at the N-terminus of an alpha-helix, thereby further stabilizing the helix. The proline derivatives, precursors prior to cross-linking, and the cross-linked peptides are provided as well as methods of using and preparing these compounds and peptides.
Owner:PRESIDENT & FELLOWS OF HARVARD COLLEGE
Stapled peptides and method of synthesis
ActiveUS8586707B2Reasonable reaction yieldsImprove stabilityPeptide/protein ingredientsPeptide sourcesCycloadditionStapled peptide
A method for preparing stapled peptides. The stapled peptides, including helical stapled peptides, are prepared according to a photochemically-based method, a [3+2] cycloaddition reaction. The helical stapled peptides exhibit increased helicity, thermal stability and cell permeability.
Owner:THE RES FOUND OF STATE UNIV OF NEW YORK
Modified therapeutic agents, stapled peptide lipid conjugates, and compositions thereof
Methods and compositions are provided for extending the half-life of a therapeutic agent. A modified therapeutic agent (mTA) comprises a therapeutic agent, a staple, and a half-life extending molecule. The mTAs disclosed herein may be used to treat a disease or a condition in a subject in need thereof.
Owner:THE SCRIPPS RES INST
Stapled peptide conjugates and particles
InactiveUS20190365913A1Reduce deliveryImproved biodistributionPowder deliveryCell receptors/surface-antigens/surface-determinantsCancer preventionDiagnostic agent
Conjugates of an active agent such as a therapeutic, prophylactic, or diagnostic agent attached to a targeting moiety via a linker, and particles comprising such conjugates have been designed which can provide improved temporospatial delivery of the active agent and / or improved biodistribution. Methods of making the conjugates, the particles, and the formulations thereof are provided. Methods of administering the formulations to a subject in need thereof are provided, for example, to treat or prevent cancer or infectious diseases.
Owner:TVA (ABC) LLC
Stapled and stitched polypeptides and uses thereof
The present invention provides stapled polypeptides of the Formulae (I) and (VI):and salts thereof; wherein the groups ; R1a, R1b, R1c, R2a, R3a, R2b, R3b, R4a, R4b, RA, RZ, L1a, L1b, L2, L3, XAA, v, w, p, m, s, n, t, and q are as defined herein. The present invention further provides methods of preparing the inventive stapled polypeptides from unstapled polypeptide precursors. The present invention further provides pharmaceutical compositions comprising a stapled polypeptide of Formula (I) or (VI), and methods of using the stapled peptides. The present invention also provides modifications of the staples post ring closing metathesis.
Owner:PRESIDENT & FELLOWS OF HARVARD COLLEGE
Cell penetrating stapled peptide, manufacturing method therefor, and use thereof
ActiveUS20170342108A1Effective penetrationReduce the numberPeptide-nucleic acidsOrganic active ingredientsStapled peptideHydrophobe
The present invention relates to a stapled peptide, a preparation method thereof and the use thereof, and more specifically to an amphipathic alpha-helical stapled peptide comprising hydrophobic amino acids and hydrophilic amino acids, a preparation method thereof, and the use thereof for intracellular delivery of an active substance.
Owner:SEOUL NAT UNIV R&DB FOUND
Stapled peptides for restraining osteoclast differentiation and preparation method and application of stapled peptides for restraining osteoclast differentiation
ActiveCN111233977AHigh purityThe method is simplePeptide/protein ingredientsSkeletal disorderStapled peptideOsteoporosis
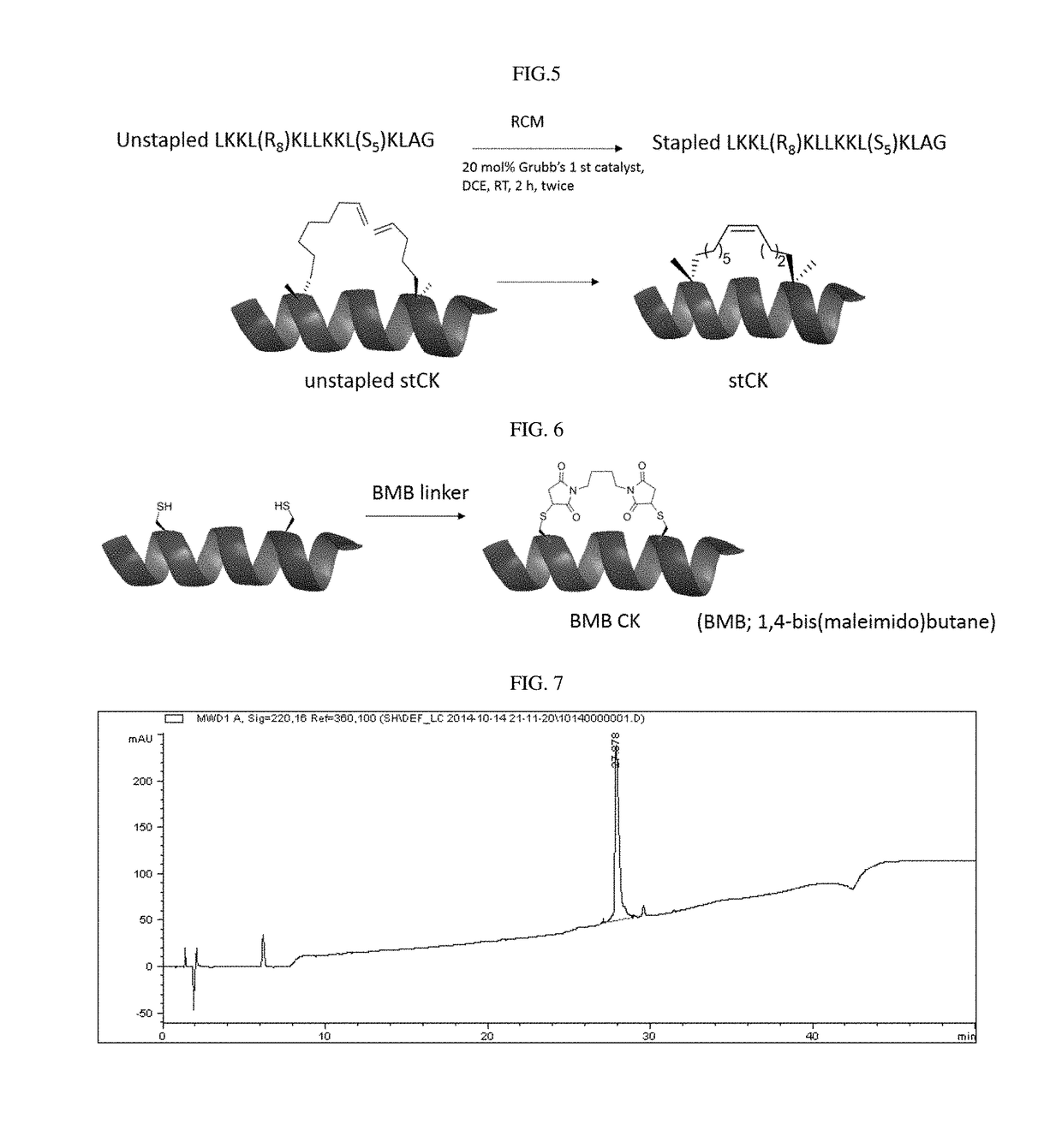
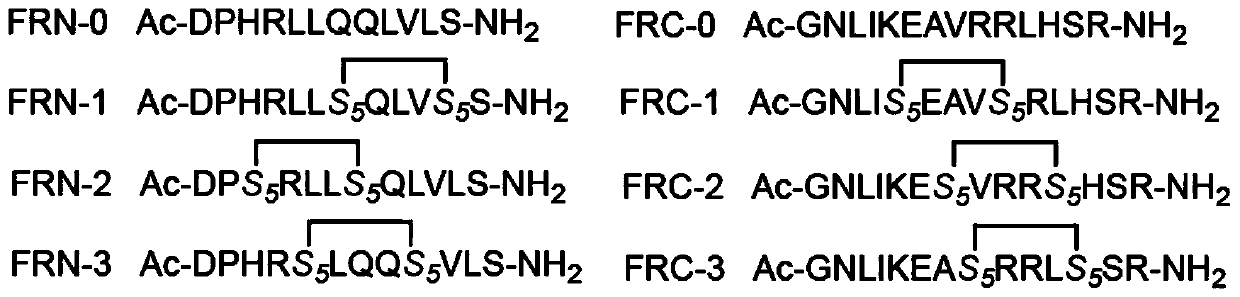
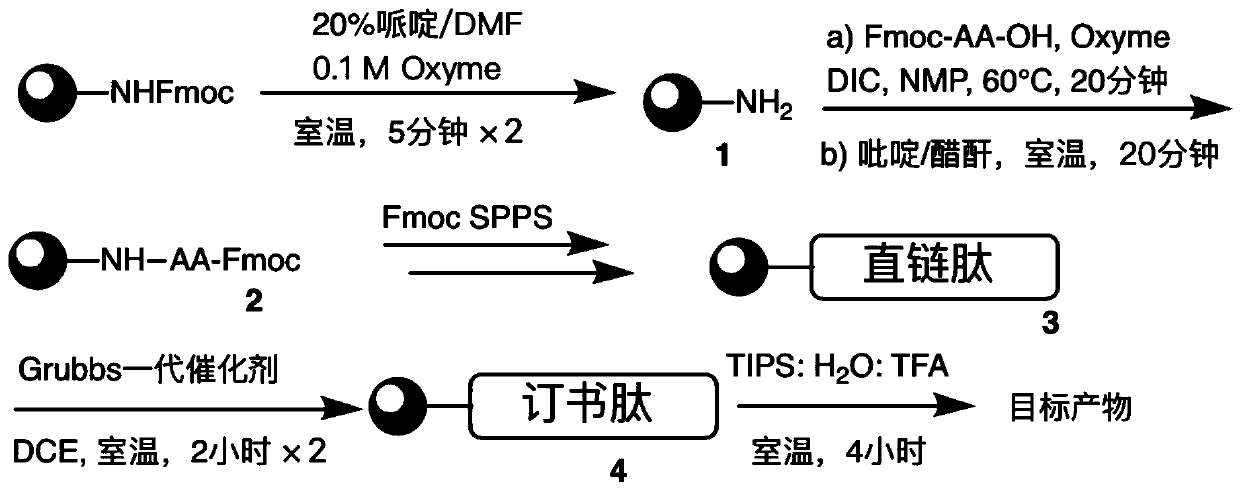
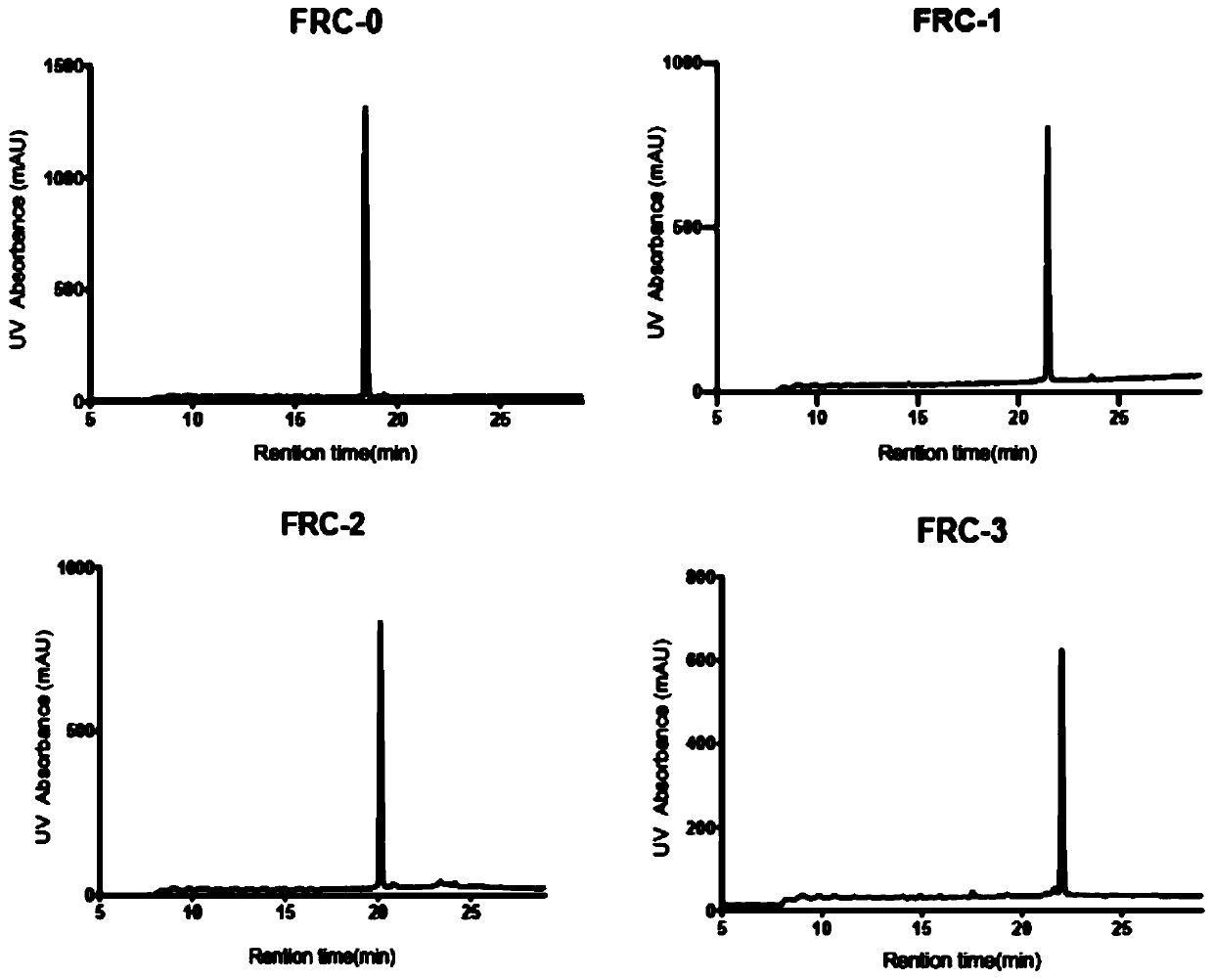
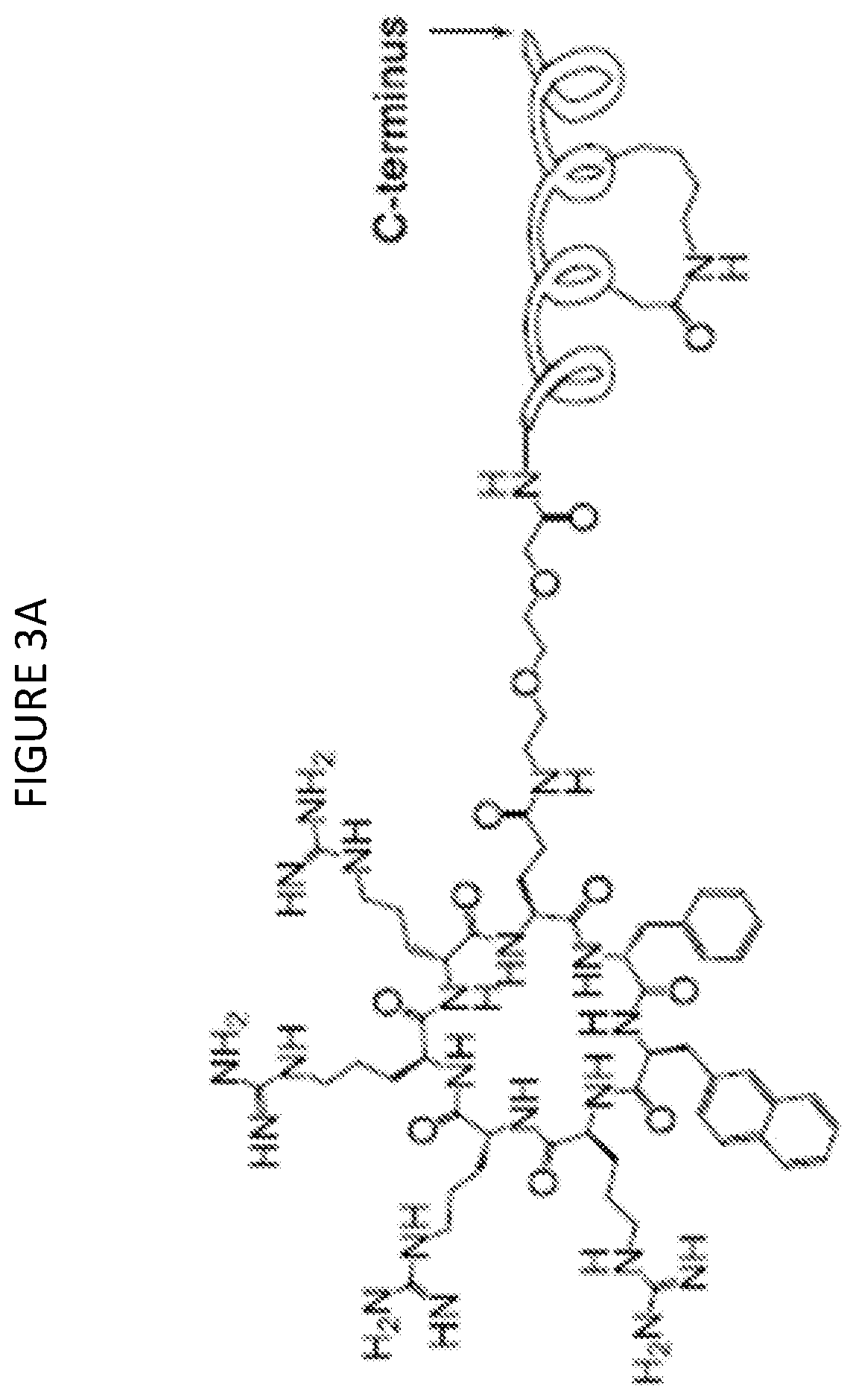
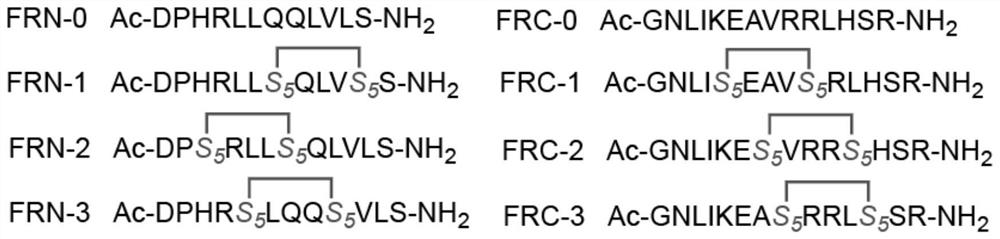
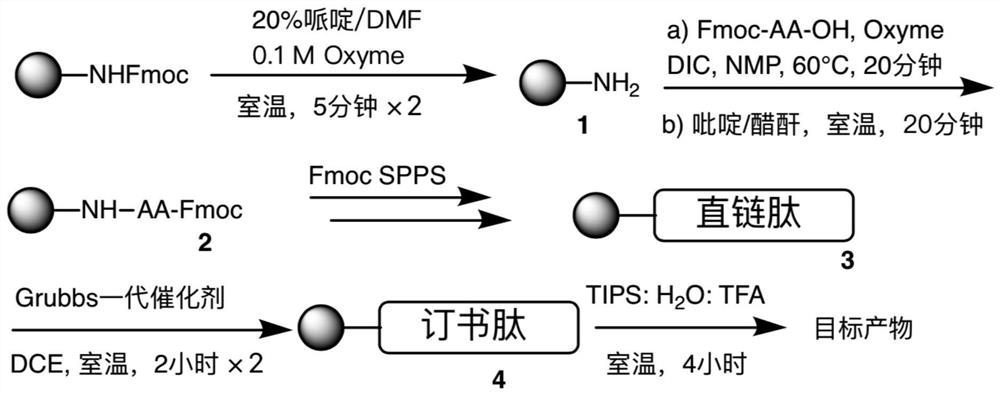
The invention relates to stapled peptides for restraining osteoclast differentiation and a preparation method and application of the stapled peptides for restraining osteoclast differentiation. Aminoresins are used as a carrier, according to the amino acid sequence of a formwork FRATtide:Ac-DPHRLLQQLVLSGNLIKEAVRRLHSR-NH2 in a DIC-Oxime condensation system, through an Fmoc solid phase synthesis method, a peptide chain is obtained by synthetizing, based on reserving key amino acid residues, S5 is used for replacing original amino acid in the specific positions, and liner-chain peptides connected to the resin are subjected to olefin metathesis reaction and cyclization in a dichloroethane solution of a Grubbs I reagent, and then are cut down from the resin, so that the target stapled peptidesare obtained. The method disclosed by the invention is simple to realize, and high in production rate, and the stapled peptides are high in purity. Further experiment confirms that the stapled peptides can notably restrain osteoclast differentiation, and have potential application value in treatment of relevant diseases of osteoporosis and the like.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
Stapled and stitched polypeptides and uses thereof
Owner:PRESIDENT & FELLOWS OF HARVARD COLLEGE
Magnetic resonance imaging compound, magnetic resonance imaging agent and application thereof as well as magnetic resonance imaging method
ActiveCN107663228AIncrease contrastEasy diagnosisPeptidesIn-vivo testing preparationsResonanceCell membrane
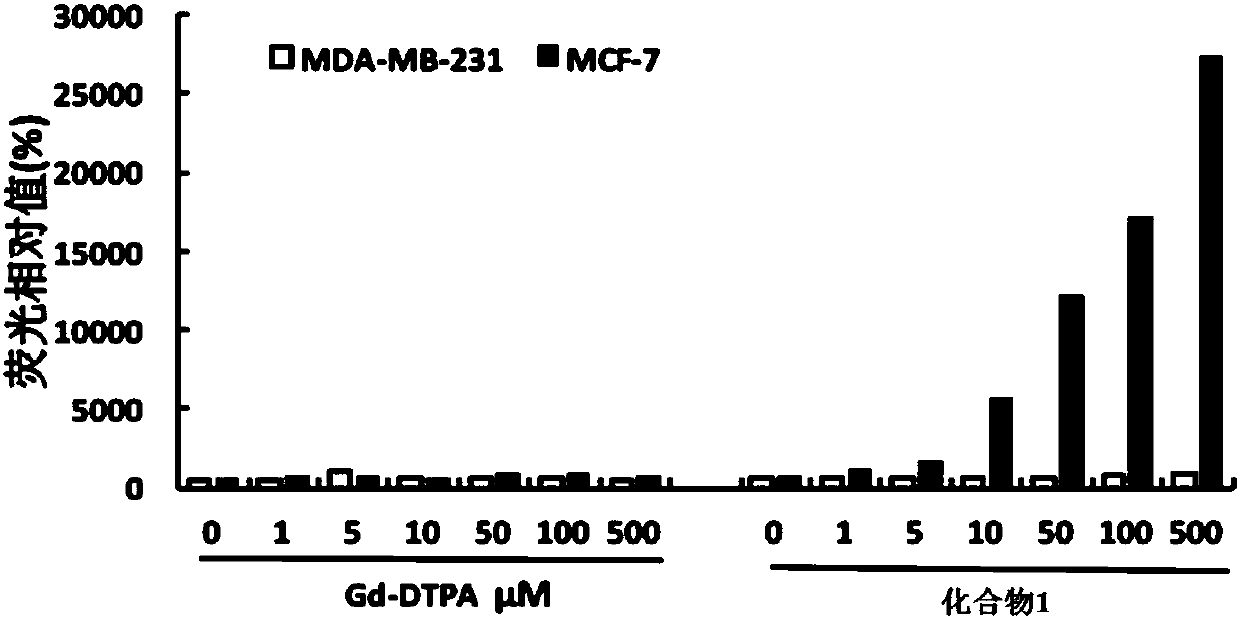
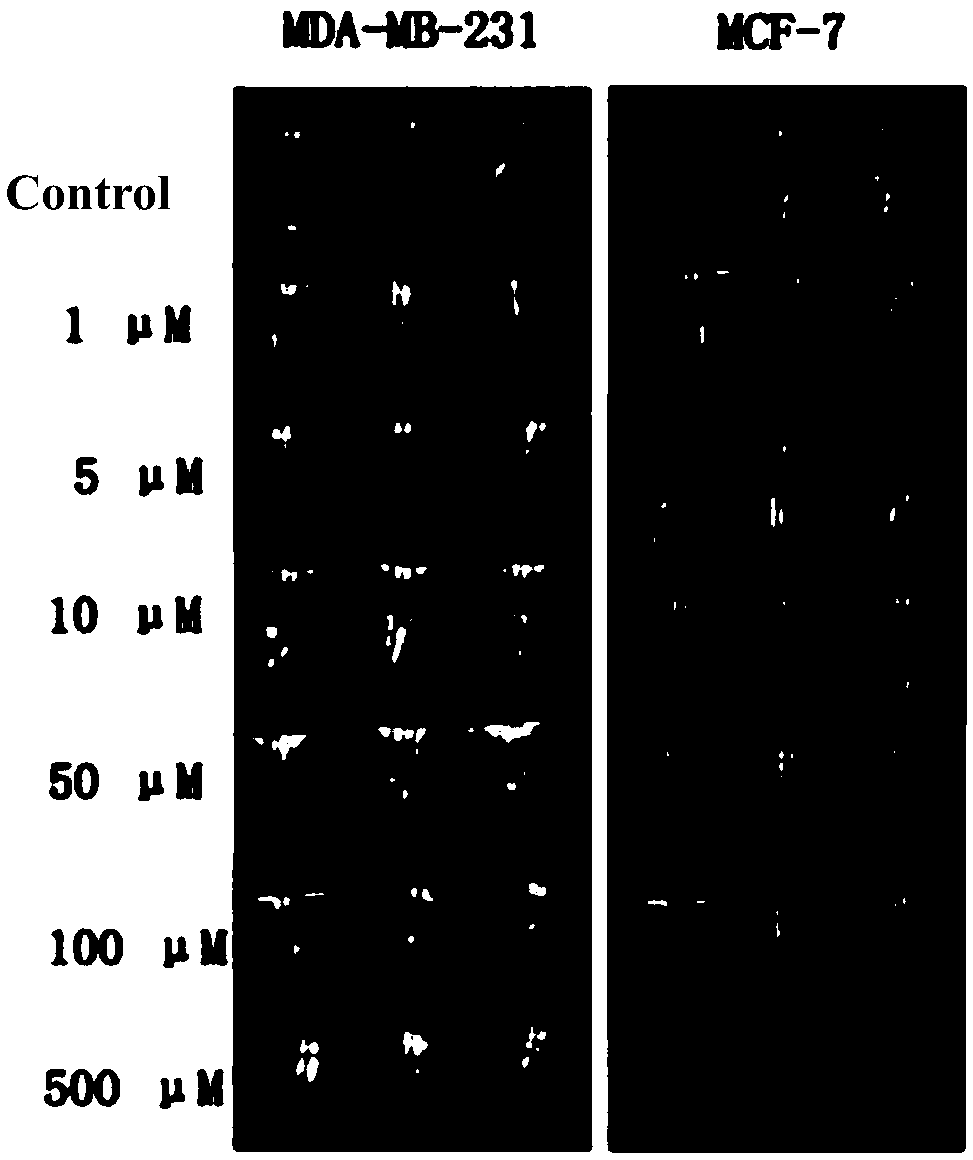
The invention relates to a stapled peptide type compound or pharmaceutically acceptable salt thereof, a magnetic resonance imaging agent and application thereof as well as a magnetic resonance imagingmethod. The stapled peptide type compound has a general formula structure shown in a formula (I), wherein R1, R2, R3, Ln and Xaa are defined in the specification. The stapled peptide type magnetic resonance imaging compound can penetrate a cell membrane, and enter a cell nucleus to be combined with an estrogen receptor located in the cell nucleus, and intracellular imaging is carried out specifically and selectively in a targeting manner. (The formula (I) is described in the specification).
Owner:BEIJING NEUROSURGICAL INST
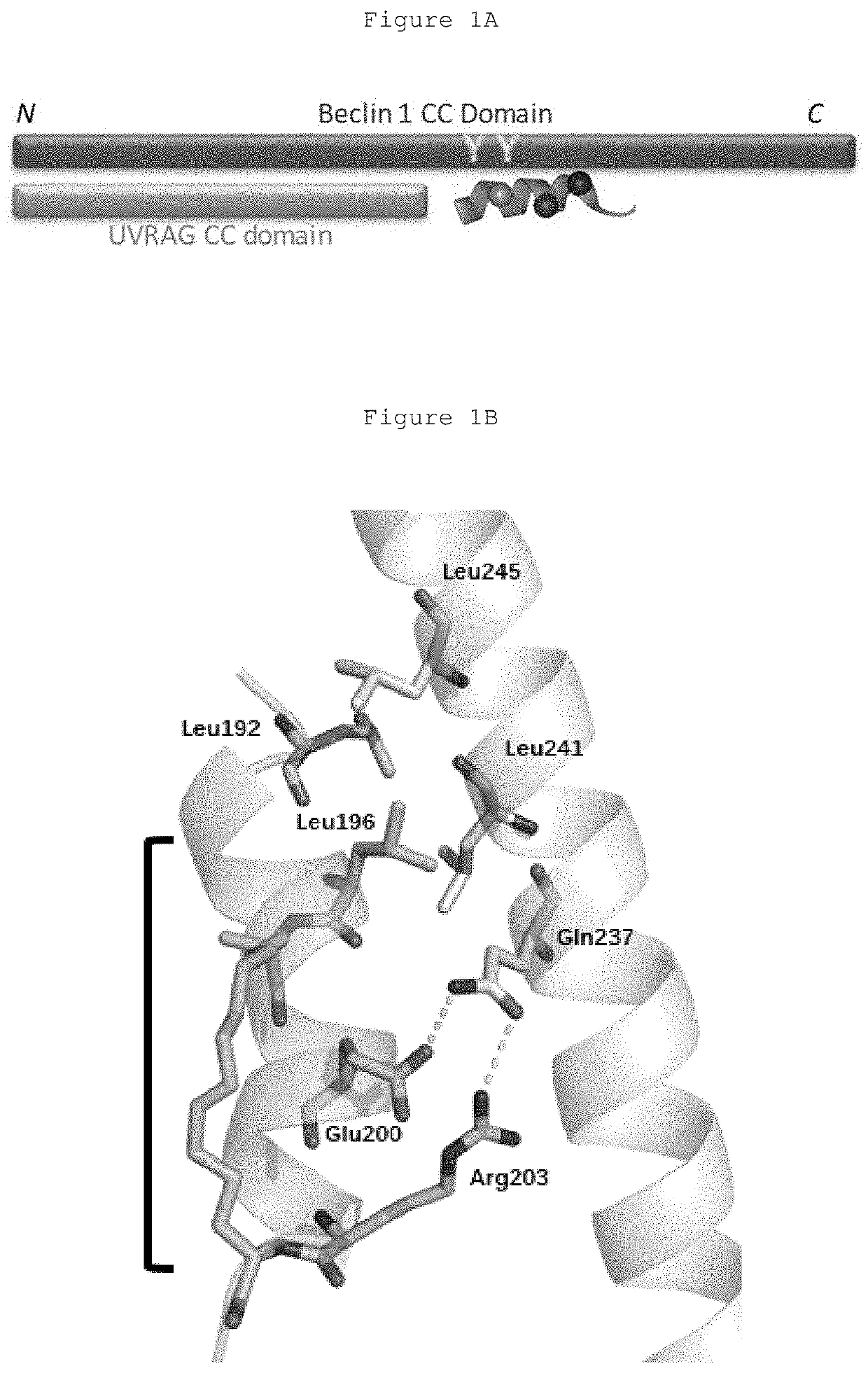
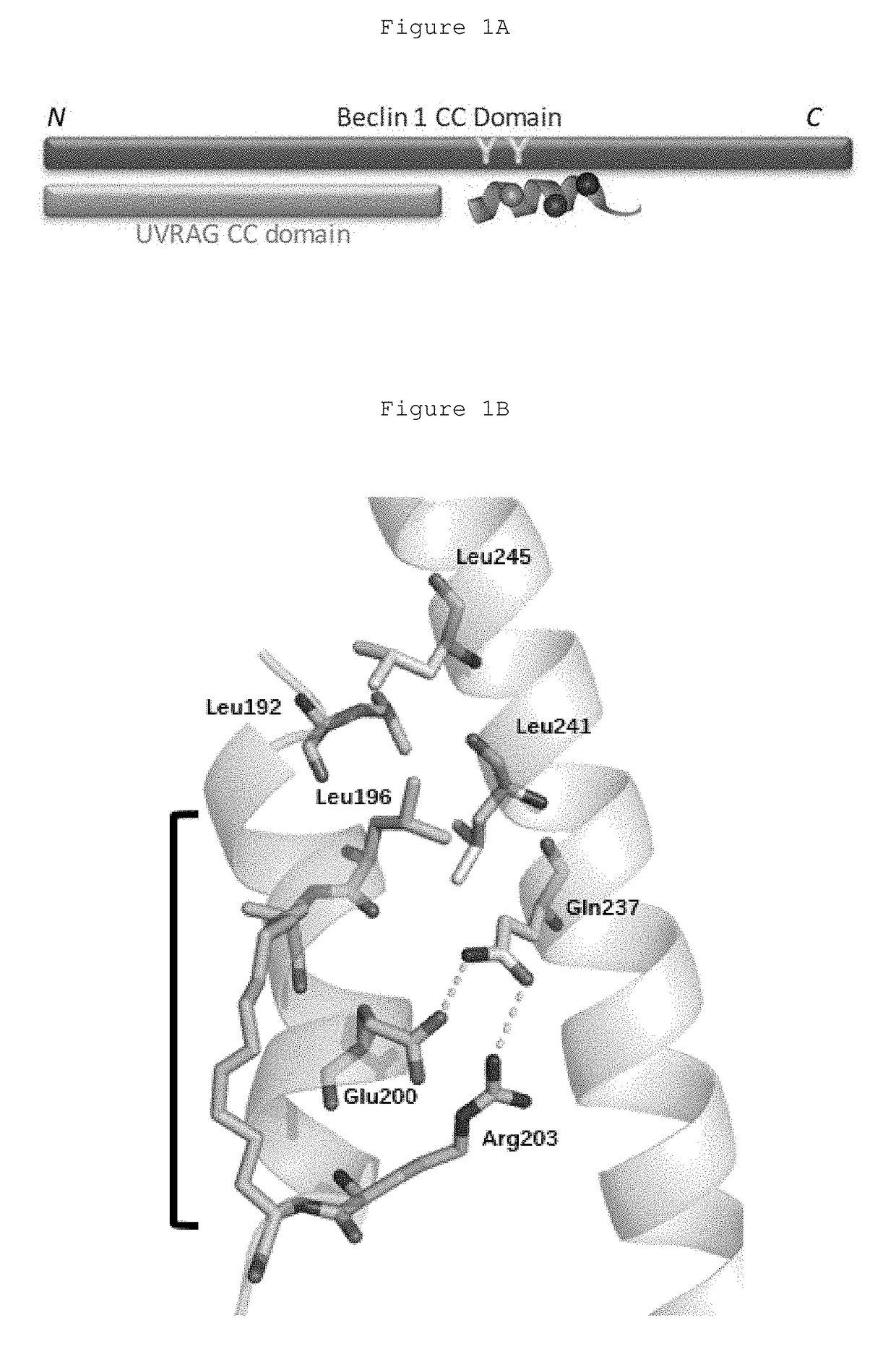
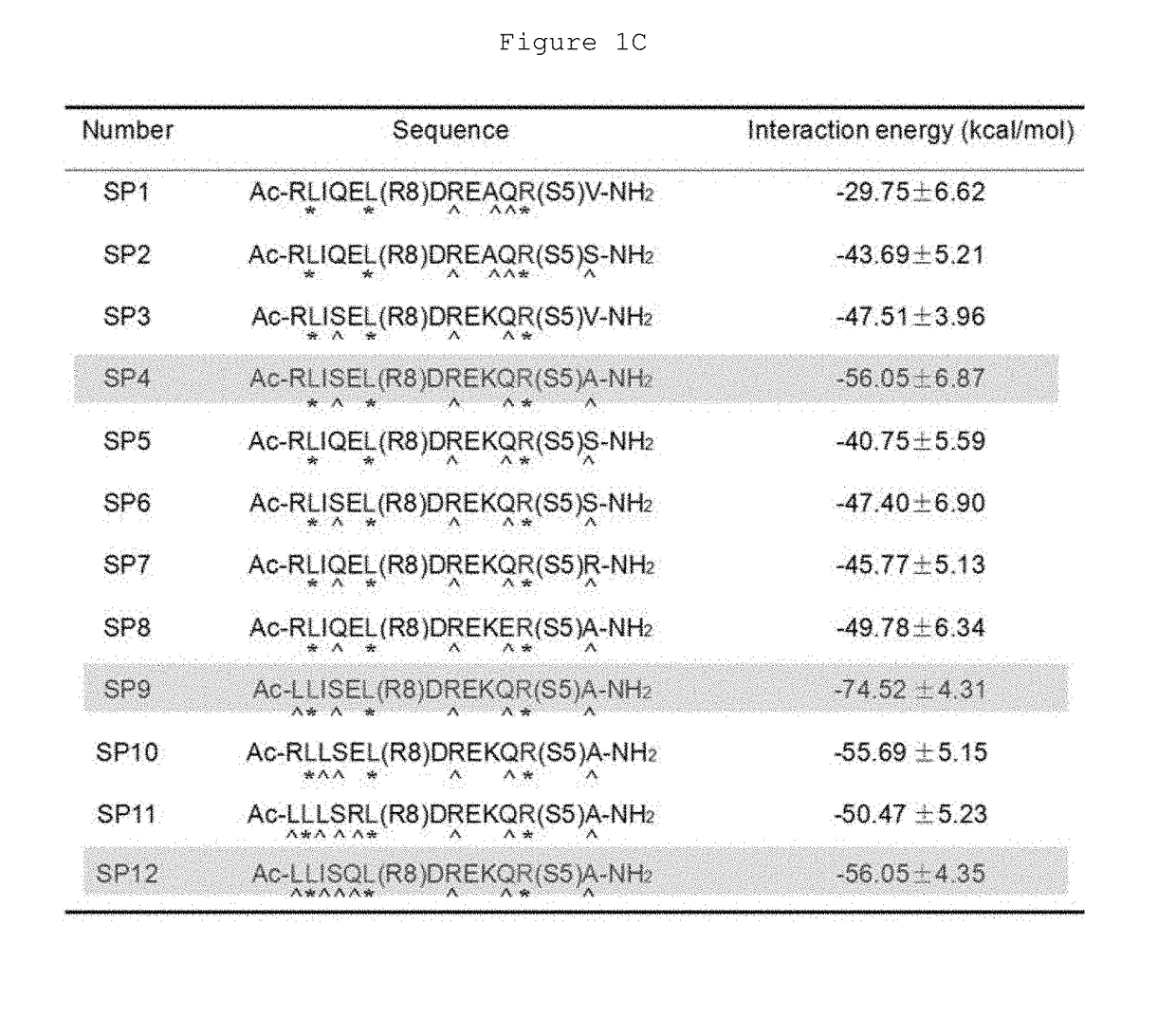
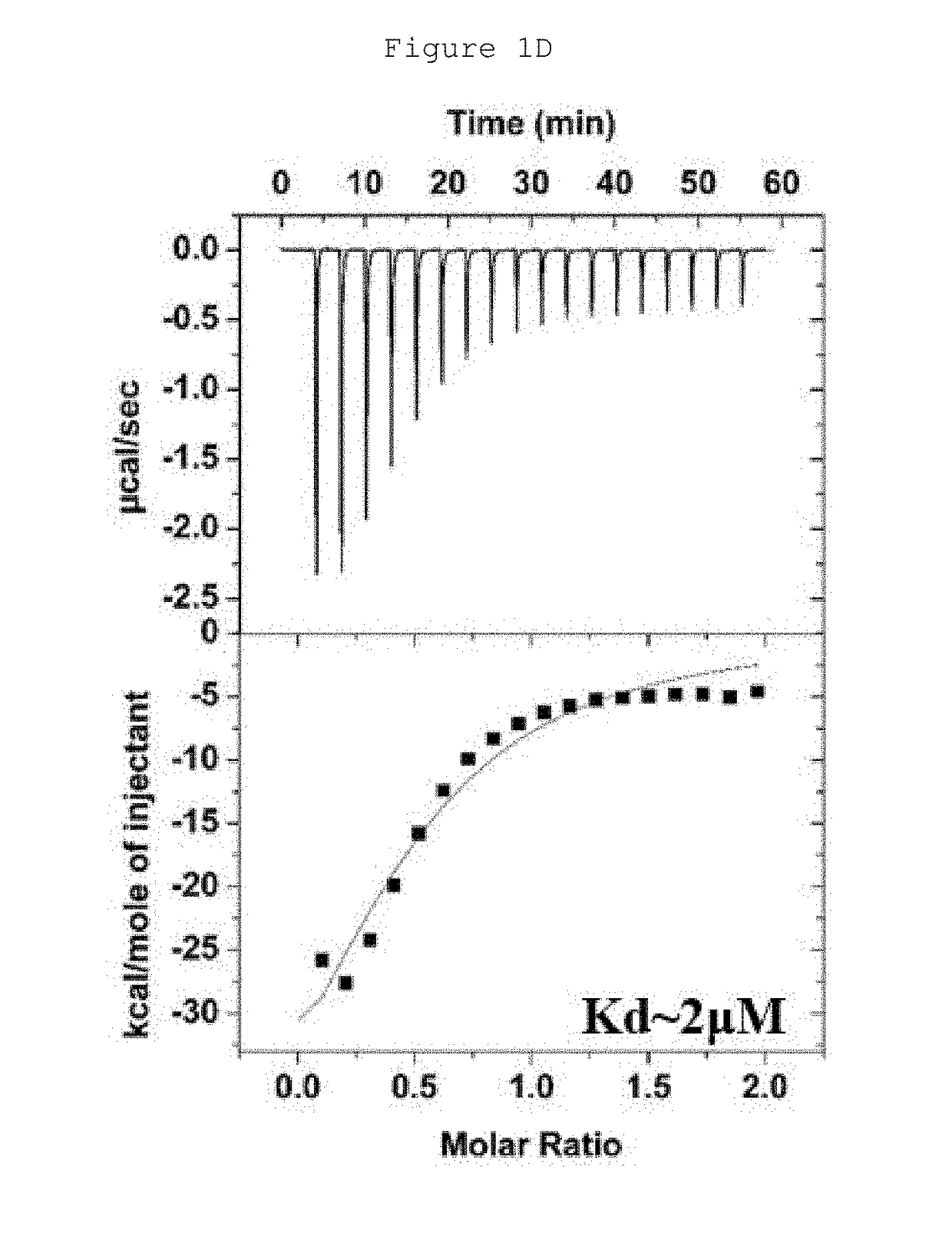
Hydrocarbon-stapled polypeptides for enhancement of endosome-lysosomal degradation
InactiveUS20180002381A1High trafficExcessive degradationPeptide/protein ingredientsApoptosis related proteinsAutophagic deathPhysical interaction
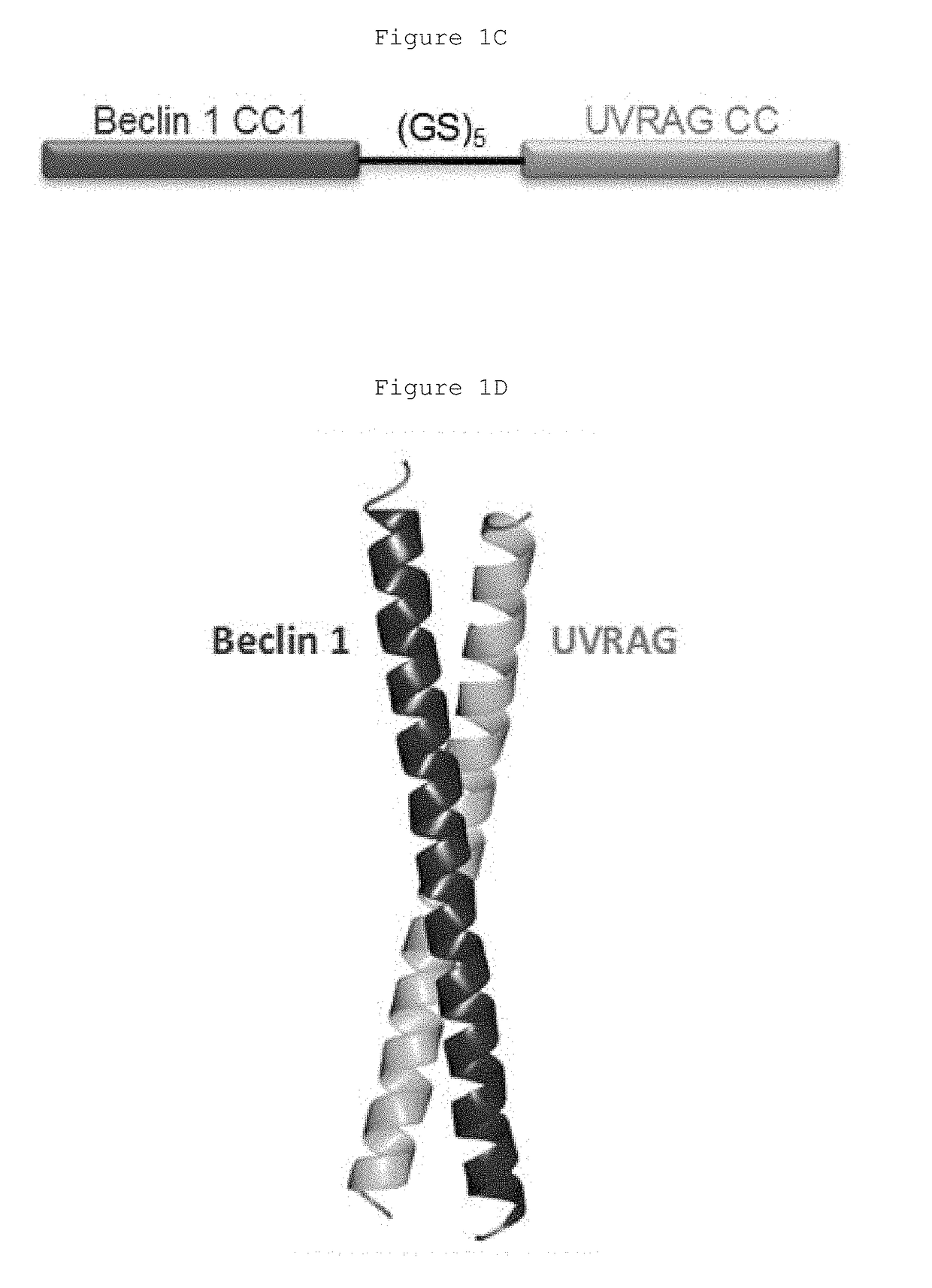
The present invention relates to a Beclin1-UVRAG complex structure which reveals a tightly packed coiled coil assembly with Beclin 1 and UVRAG residues complementing each other to form a stable dimeric complex. This potent physical interaction is critical for UVRAG-dependent EGFR degradation but less critical for autophagy. Targeting the Beclin coiled coil domain with rationally designed stapled peptides leads to enhanced autophagy activity and EGFR degradation in non-small cell lung cancer (NSCLC) cell lines, suggesting translational value for these compounds.
Owner:THE HONG KONG POLYTECHNIC UNIV
Novel stapled peptides serving as KRASG12C/SOS1 inhibitor and application of novel stapled peptide
ActiveCN111393519AHigh alpha-helix contentΑ-helix content maintenancePeptide/protein ingredientsRespiratory disorderChymotrypsinStapled peptide
The invention discloses novel stapled peptides serving as a KRASG12C / SOS1 inhibitor and application of the novel stapled peptides. Also provided are a composition comprising these stapled peptides anda method for using such peptides in the treatment of cancers. The stapled peptides are more stable in alpha-helical conformation to some extent; the stapled peptides are higher in affinity with KRASG12C protein; the enzymolysis capability of resisting trypsin and alpha-chymotrypsin is better; besides, the plasma stability of the stapled peptides is greatly enhanced, and the stapled peptides are derived from an alpha-helical binding region of SOS1 protein and KRAS protein and inhibit the activation of the KRAS protein. The invention also relates to a preparation method of the novel stapled peptides, a pharmaceutical composition containing these stapled peptides, and application of the stapled peptides independently used or in combination with other compounds for the prevention or treatmentof cancers (such as non-small cell lung cancer).
Owner:CHINA PHARM UNIV
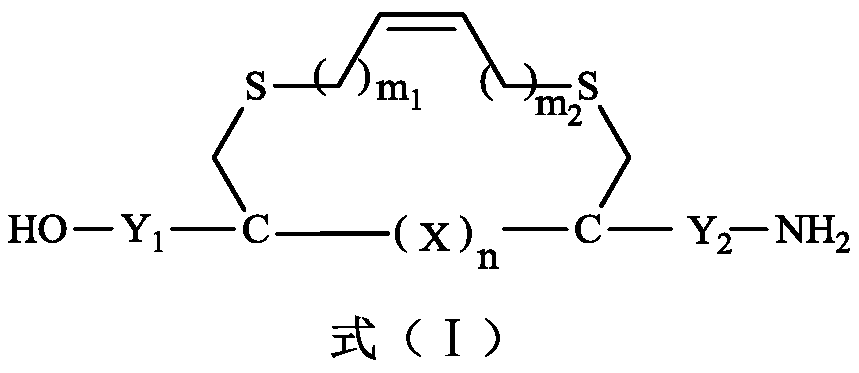
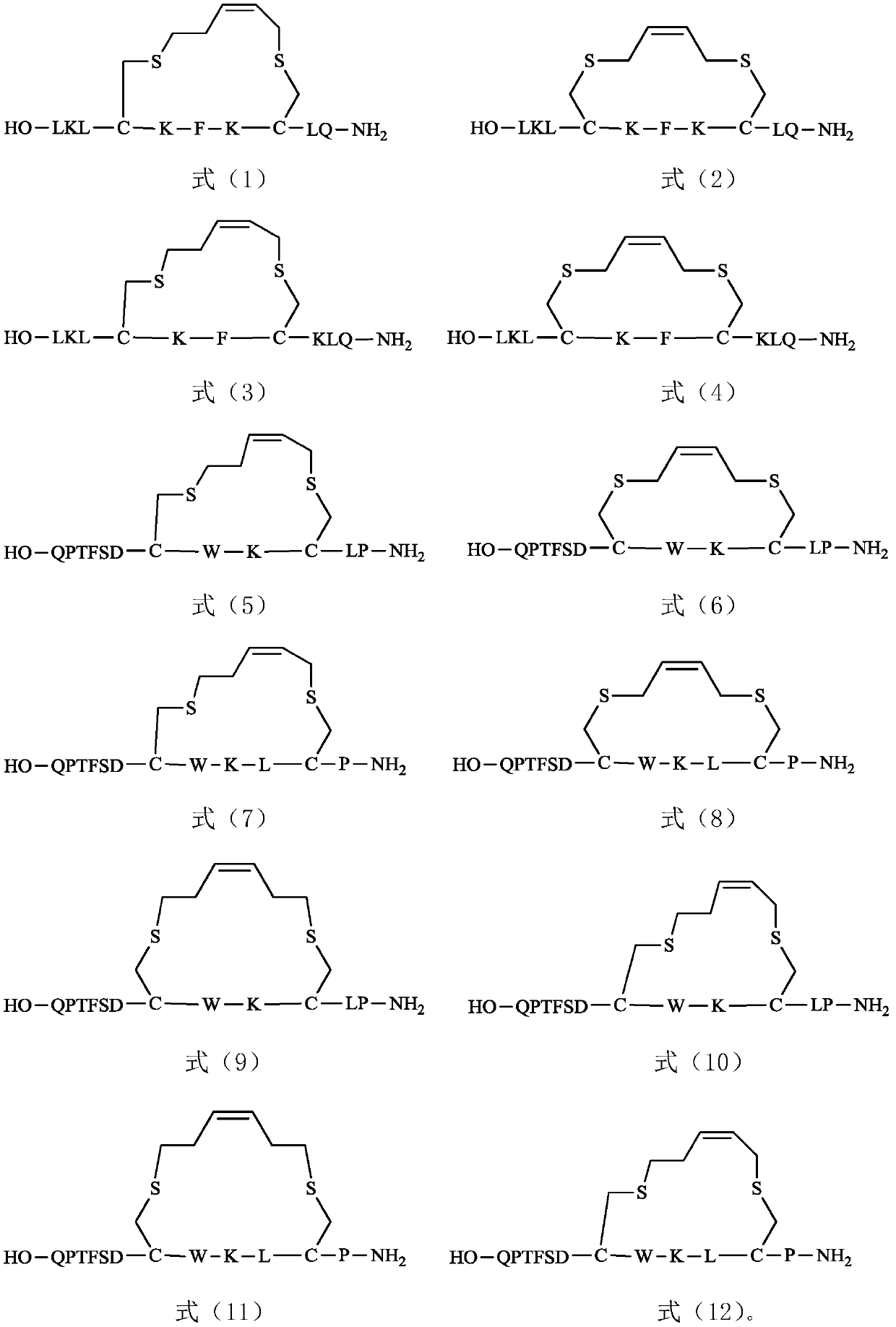
Olefin thioether stapled peptide and preparation method and application thereof
ActiveCN111040020AEasy to synthesizeDoes not affect efficacyPeptide/protein ingredientsPeptide preparation methodsCombinatorial chemistryStapled peptide
The invention discloses an olefin thioether stapled peptide. The olefin thioether stapled peptide is characterized in that the structure of the olefin thioether stapled peptide is represented by a formula (I) which is shown in the specification, wherein X is a polypeptide composed of n natural amino acids, n is an integer in the range of 2-4, m1 and m2 are integers in a value range of 1-3, C is analpha-carbon atom in cysteine, and Y1 is a polypeptide composed of 3-8 natural amino acids, and preferably, Y2 is a polypeptide composed of 2-5 natural amino acids or any one of the natural amino acids. The stapled peptide molecules show obvious anti-proliferative activity in different tumor cells, and can be used for treating tumor diseases.
Owner:ACADEMY OF MILITARY MEDICAL SCI
Cationic bridged stapled peptide and application thereof
PendingCN110627871AImprove stabilityHigh antibacterial activityAntibacterial agentsPeptide/protein ingredientsResistant bacteriaCrystallography
The invention discloses a cationic bridged stapled peptide. The stapled peptide is a cyclic polypeptide with 10-20 amino acid residues, and has the following sequences and structural characteristics:a polypeptide sequence can be shown as a general formula P<n>-K<c>-P<m>-K<c>-P, wherein the P<n>, the P<m> and the P are polypeptide fragments with n, m and i amino acid residues, the n and thei are natural numbers, the m is 3 or 6, the K represents lysine, and the c represents that a cyclic structure is formed between two lysine side chain amino groups. The invention provides a preparationmethod and application of the cationic bridged stapled peptide. A cyclic structure is innovatively formed on one side of the hydrophilic surface of amphiphilic cationic antibacterial peptide, and thestaple antibacterial peptide is prepared. The antibacterial peptide has the following advantages: the antibacterial peptide has broad-spectrum antibacterial activity, and especially has a significantinhibition effect on clinical drug-resistant bacteria; the structure is simple and synthesis is easy; the biological stability is good; the antibacterial peptide can be applied to prevention and treatment of human or animal infectious diseases or tumors.
Owner:CHONGQING UNIV
Polypeptide conjugates for intracellular delivery of stapled peptides
The present disclosure provides novel polypeptide conjugates. The polypeptide conjugates disclosed herein comprise a stapled peptide comprising a peptide and at least one staple which holds the peptide in an alpha-helical confirmation, and a cyclic cell-penetrating peptide (cCPP) conjugated, directly or indirectly, to the stapled peptide. The present disclosure demonstrates that cCPPs can be usedto confer consistent cell-permeability to stapled peptides.
Owner:OHIO STATE INNOVATION FOUND
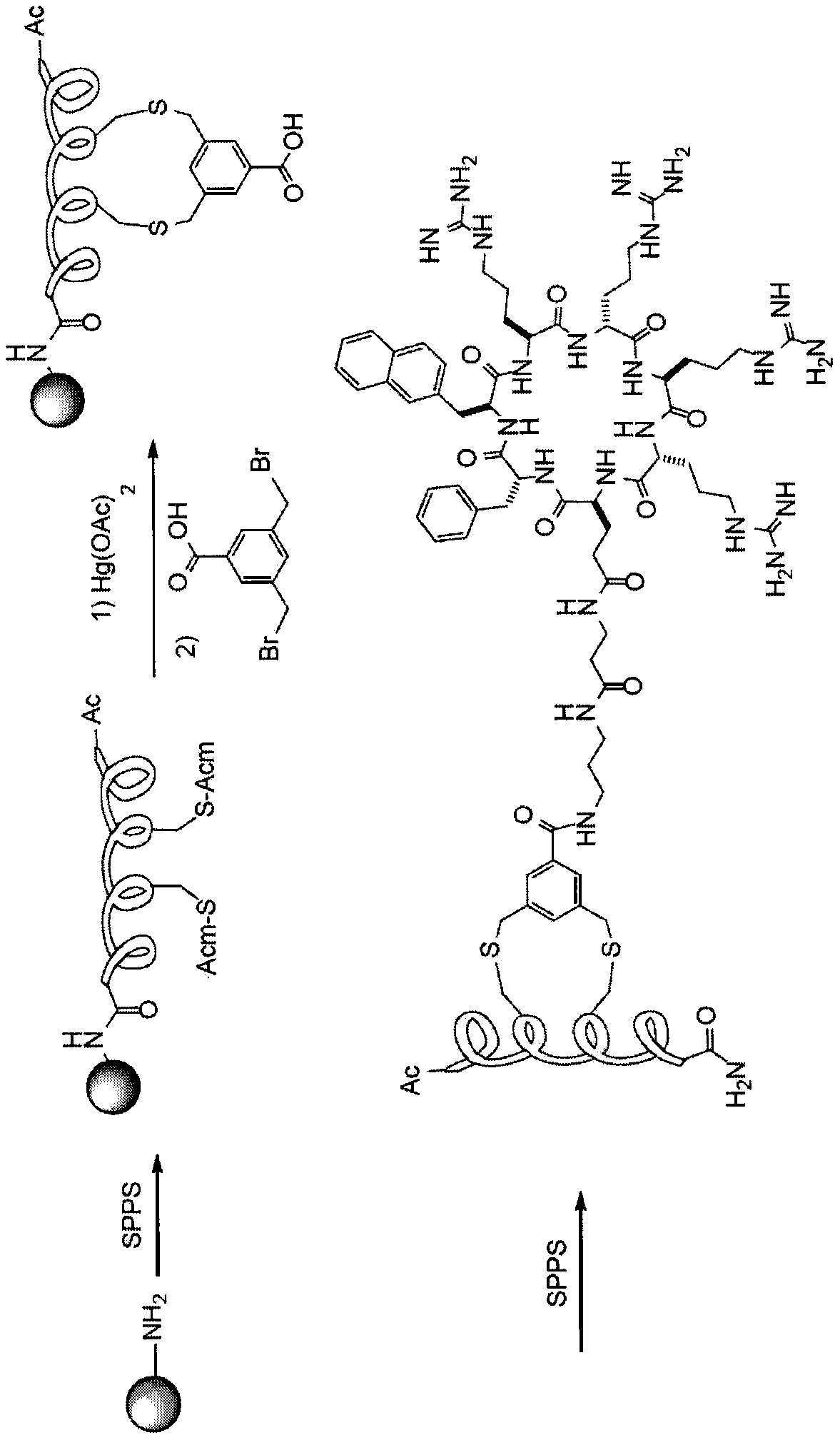
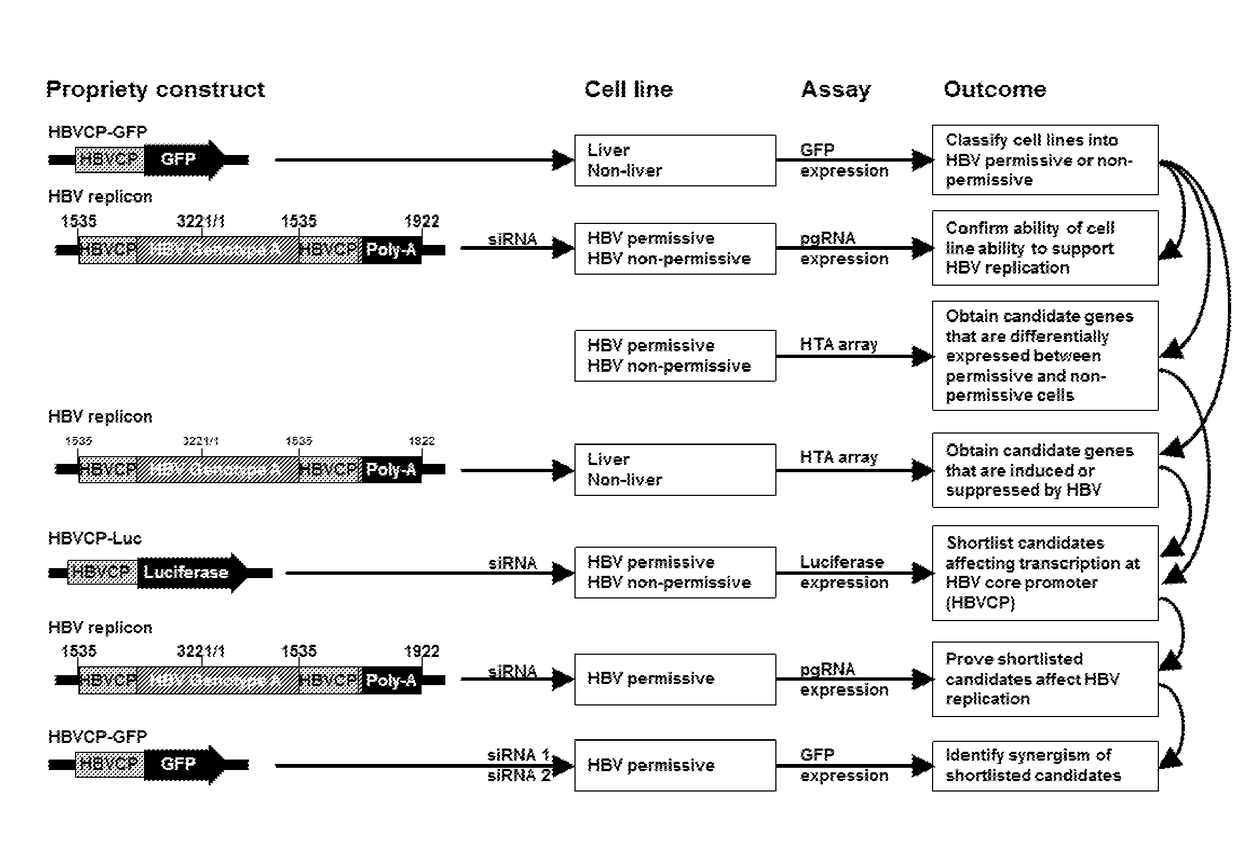
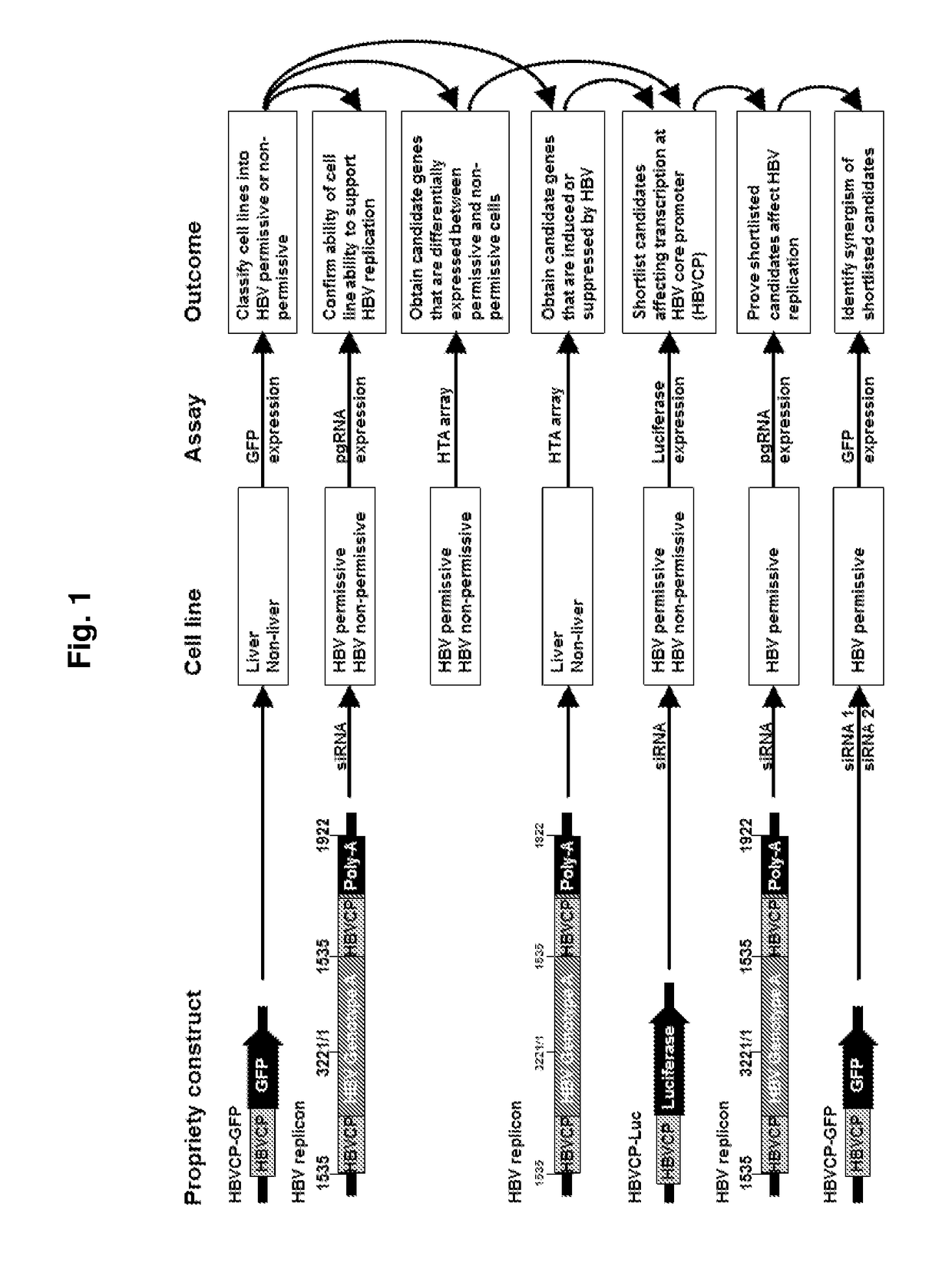
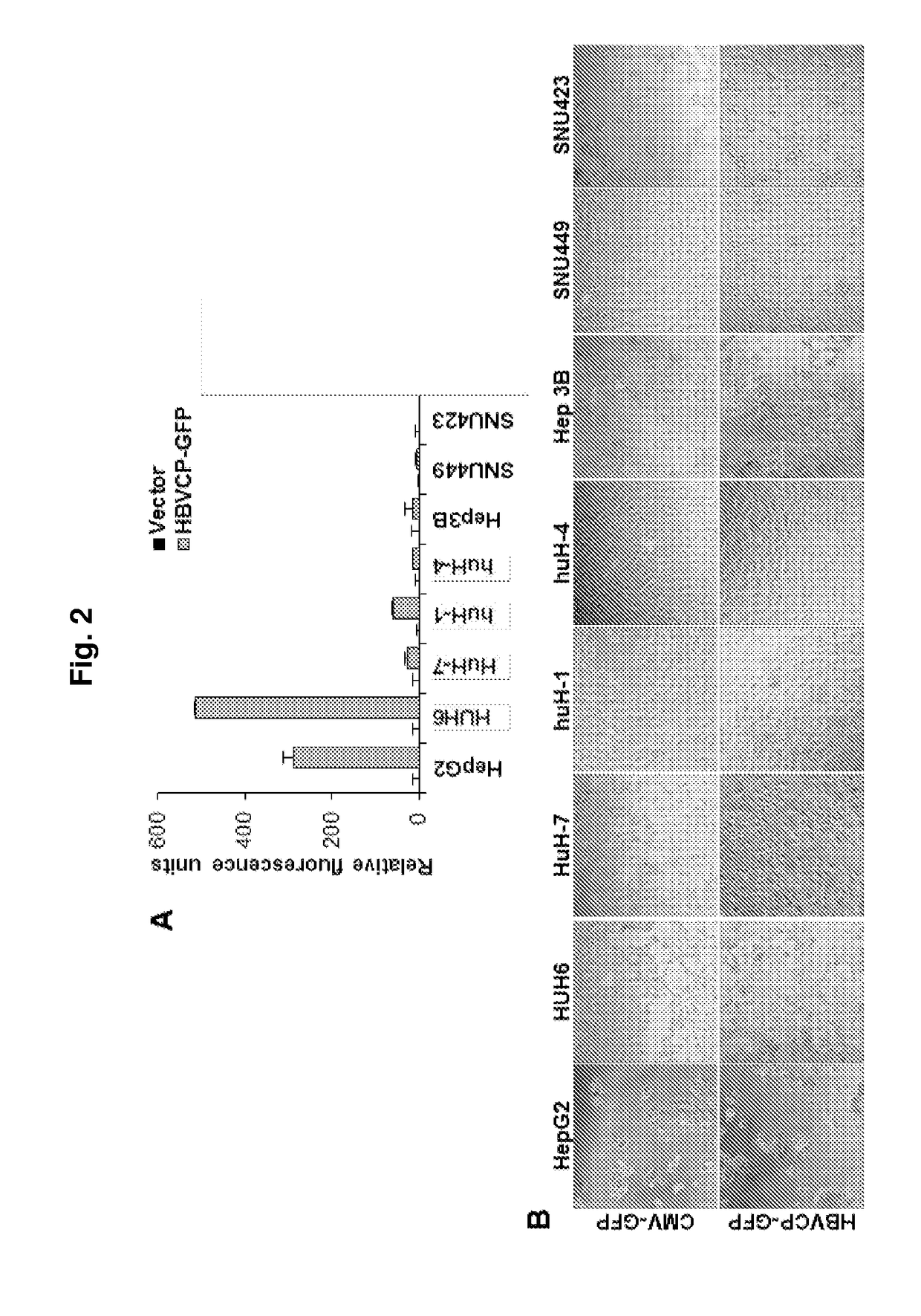
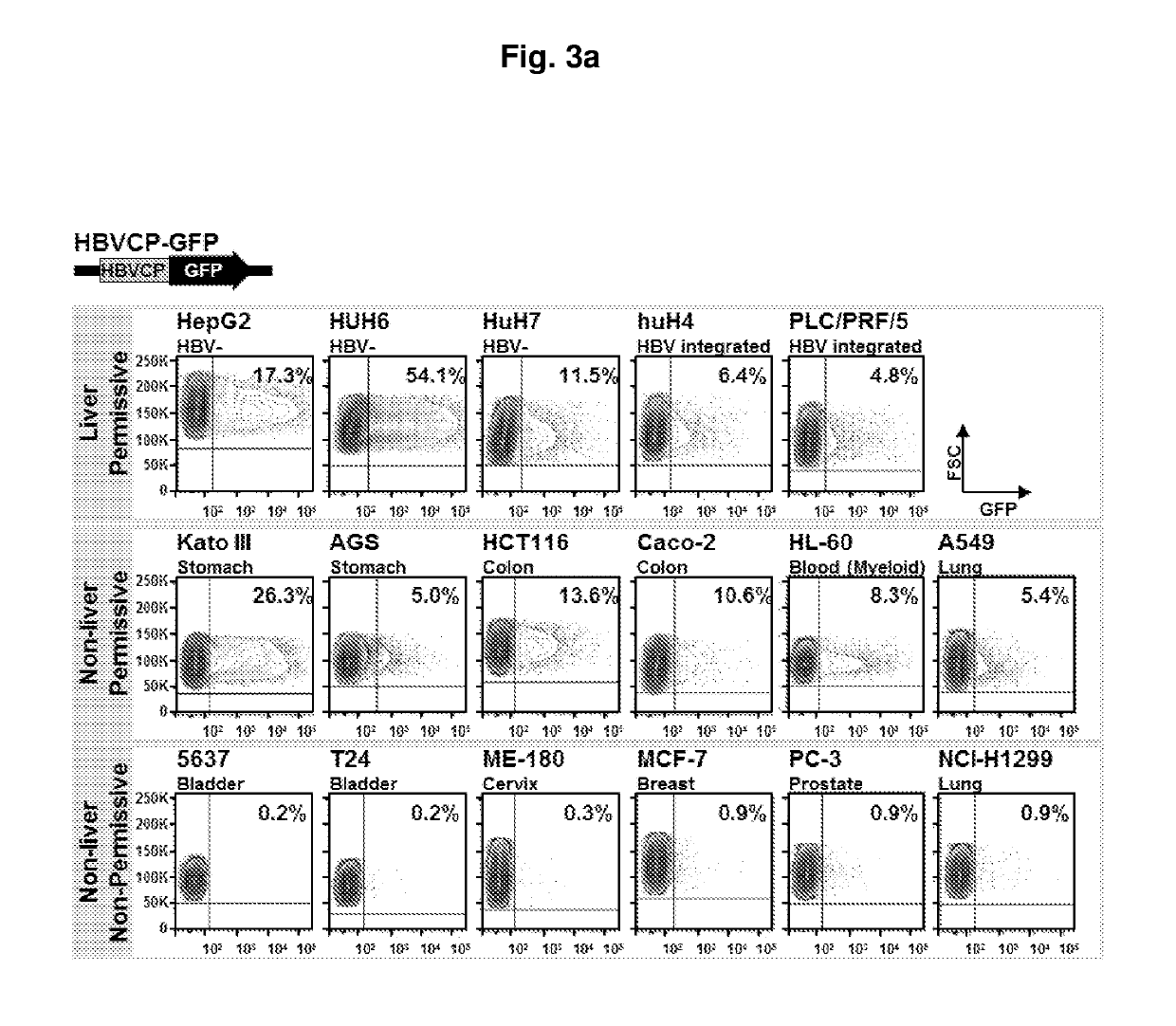
Modulation of hepatitis b virus replication
ActiveUS20180209958A1Inhibition of replicationTreat infectionOrganic active ingredientsPeptide/protein ingredientsDrugStapled peptide
Presently disclosed is a method of modulating Hepatitis B virus (HBV) replication, by contacting the cell with at least one agent that modulates at least one factor from a specified group consisting of SNAI2, SOX7 and other factors, the screening of said agent and use thereof in a medicament for treating HBV infection or disease or condition associated with a HBV infection in a subject. In one preferred embodiment, the agent is one peptide derived from SOX7 or SNAI2 or stapled peptides thereof. As a separate invention, a method of identifying at least one factor that modulates replication of a virus is also disclosed.
Owner:AGENCY FOR SCI TECH & RES
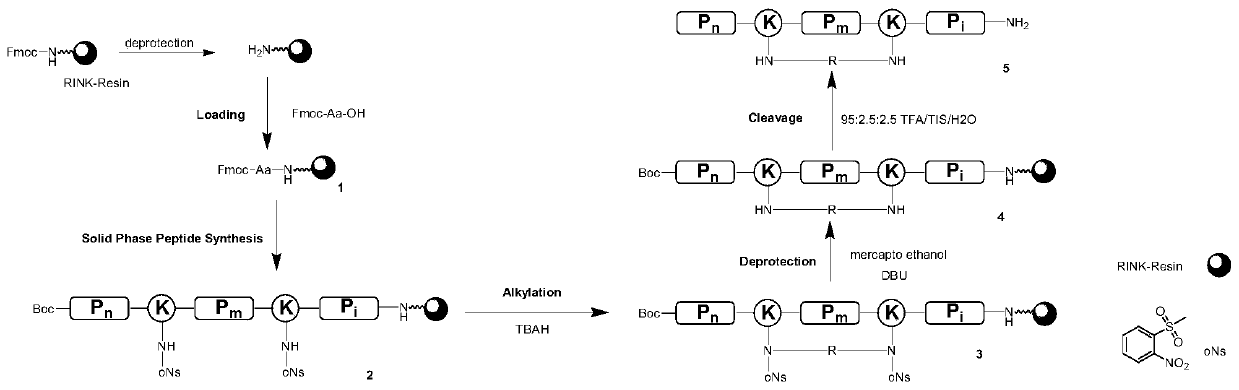
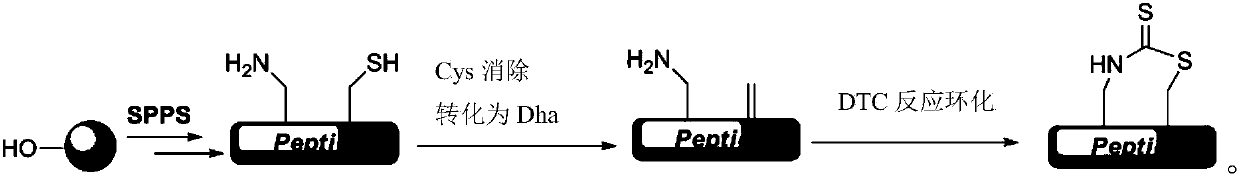
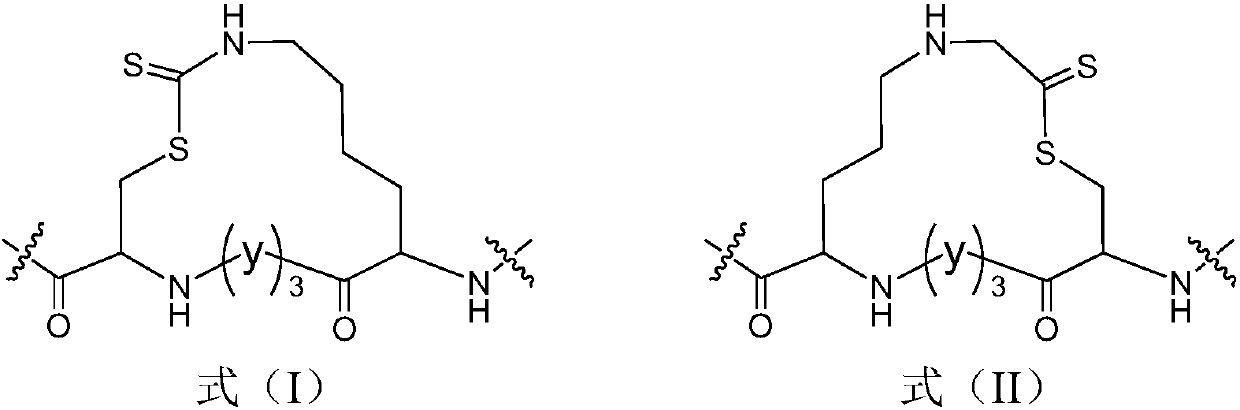
Thioester stapled peptide, preparation method and application thereof
ActiveCN107602666AStabilizes the α-helical conformationAvoid isomerizationPeptide/protein ingredientsPeptide preparation methodsSolubilityIsomerization
The invention discloses a thioester stapled peptide. The thioester stapled peptide is characterized in that a peptide chain contains segments shown as a formula (I) or a formula (II) in the description, wherein the segments are positioned at end parts or middle parts of the peptide chain. In the formula (I) and the formula (II), each y is separately selected from one of 20 protein amino acids. Theinvention further discloses a method for preparing the thioester stapled peptide. According to the method, DTC (Dithiocarbamates) synthesis reaction is introduced into the preparation of an alpha-helical conformation-locked polypeptide, an dithiocarbamate link arm is formed on a side chain of a peptide segment in order to stabilize the alpha-helical conformation of the polypeptide and fulfill theaim of locking the conformation. According to the polypeptide conformation locking strategy, the problems of product isomerization, poor water solubility, dependence on solid-phase cyclization and the like can be effectively solved.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
Hydrocarbon-stapled polypeptides for enhancement of endosome-lysosomal degradation
ActiveUS10618939B2High trafficExcessive degradationPeptidesAntineoplastic agentsLysosomeChemical compound
The present invention relates to a Beclin 1-UVRAG complex structure which reveals a tightly packed coiled coil assembly with Beclin 1 and UVRAG residues complementing each other to form a stable dimeric complex. This potent physical interaction is critical for UVRAG-dependent EGFR degradation but less critical for autophagy. Targeting the Beclin 1 coiled coil domain with rationally designed stapled peptides leads to enhanced autophagy activity and EGFR degradation in non-small cell lung cancer (NSCLC) cell lines, suggesting translational value for these compounds.
Owner:THE HONG KONG POLYTECHNIC UNIV
Stapled peptides and uses thereof
ActiveUS20180170967A1Cell receptors/surface-antigens/surface-determinantsPeptide/protein ingredientsStapled peptideChemistry
Cyclized peptides derived from the hyaluronan binding region of RHAMM are provided. Pharmaceutical compositions and methods for using the peptides and pharmaceutical compositions are also provided.
Owner:LONDON HEALTH SCI CENT RES
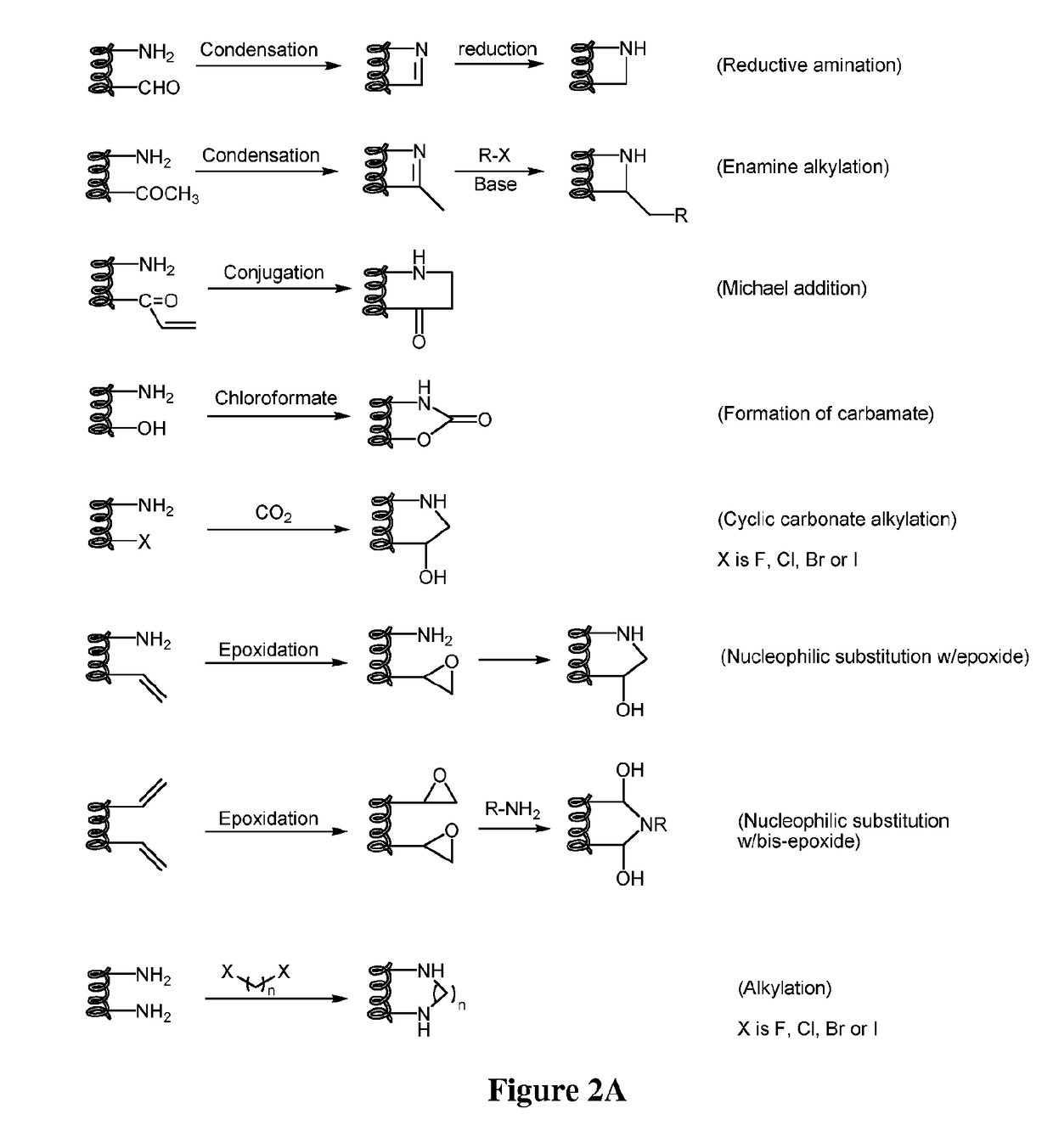
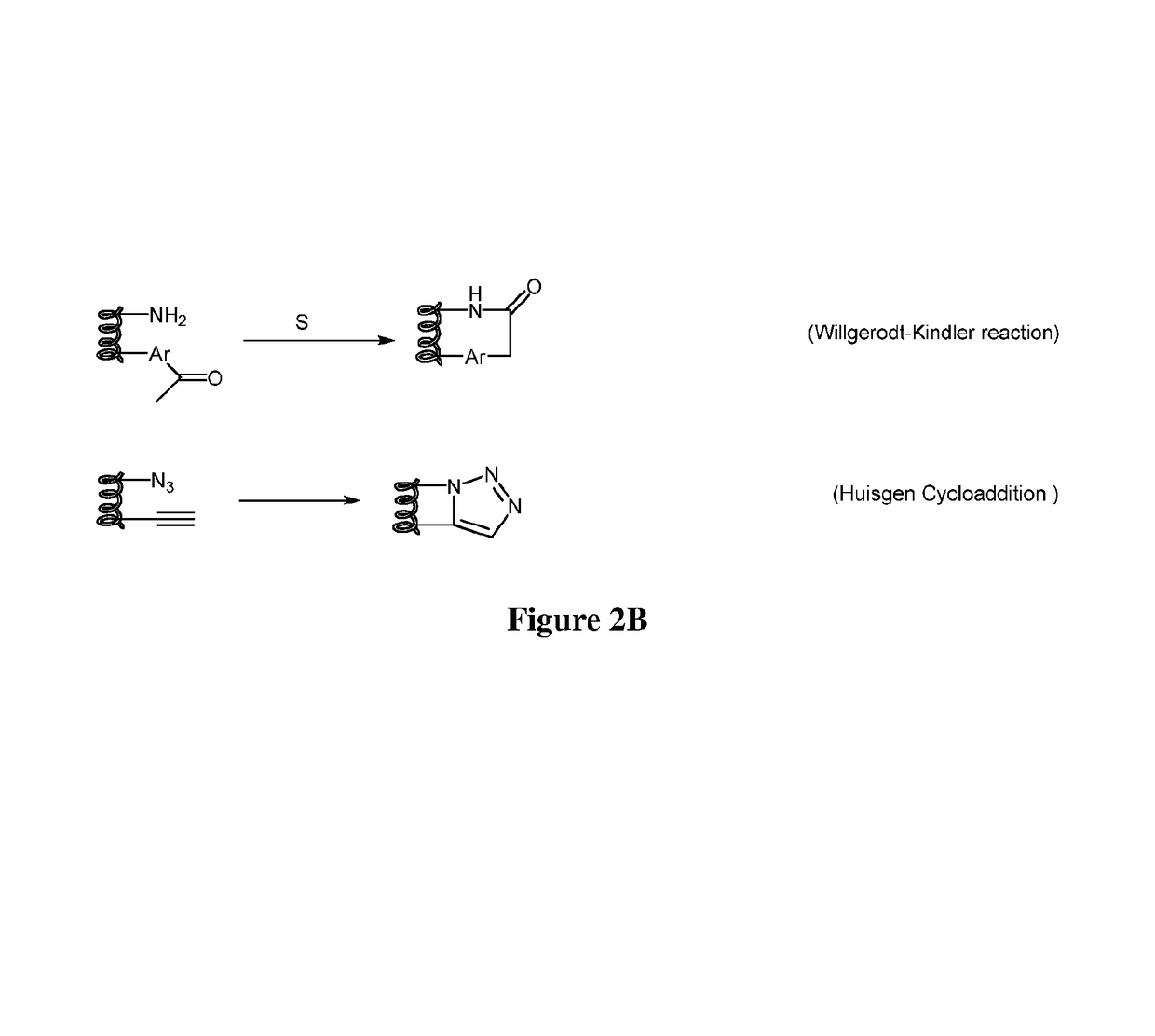
Compounds comprising stapled or stitched peptides for improved drug delivery
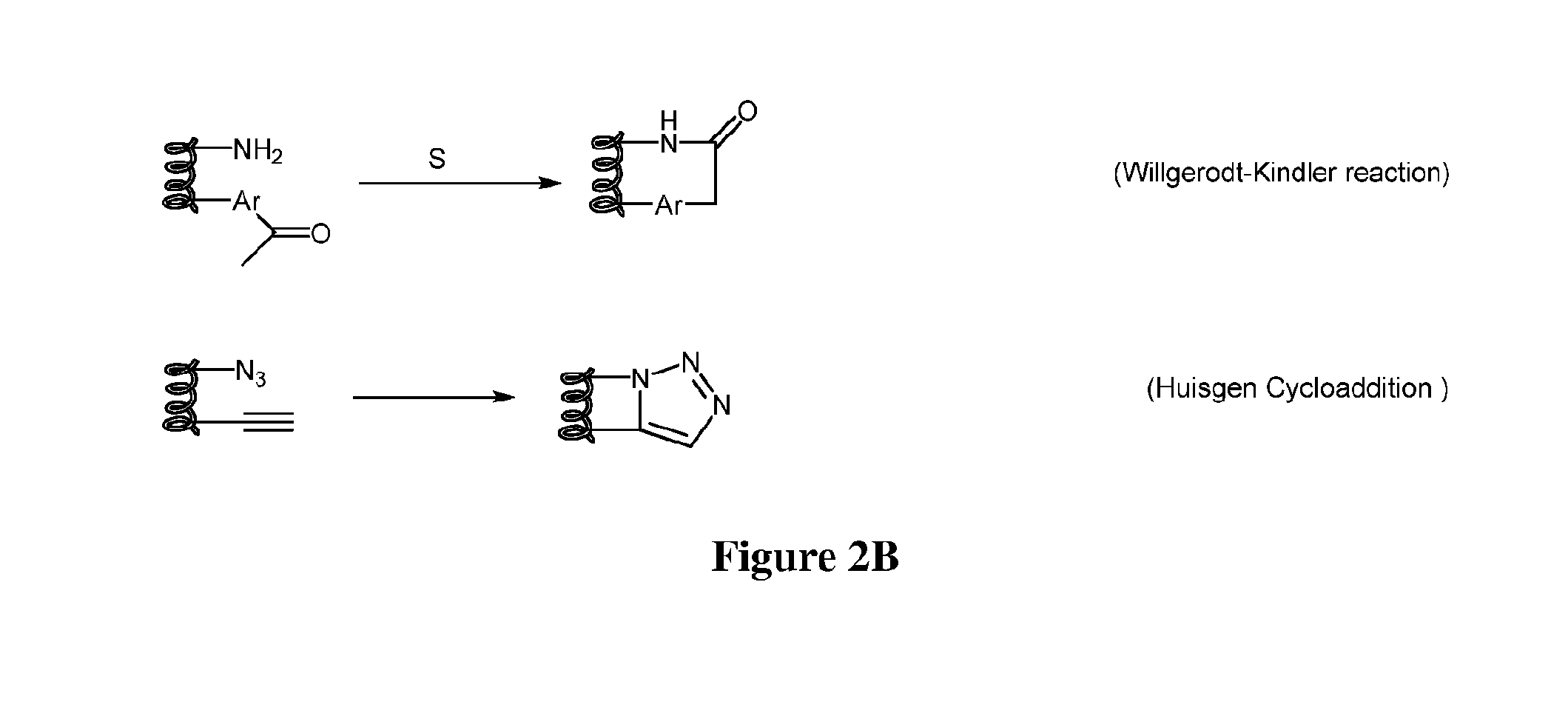
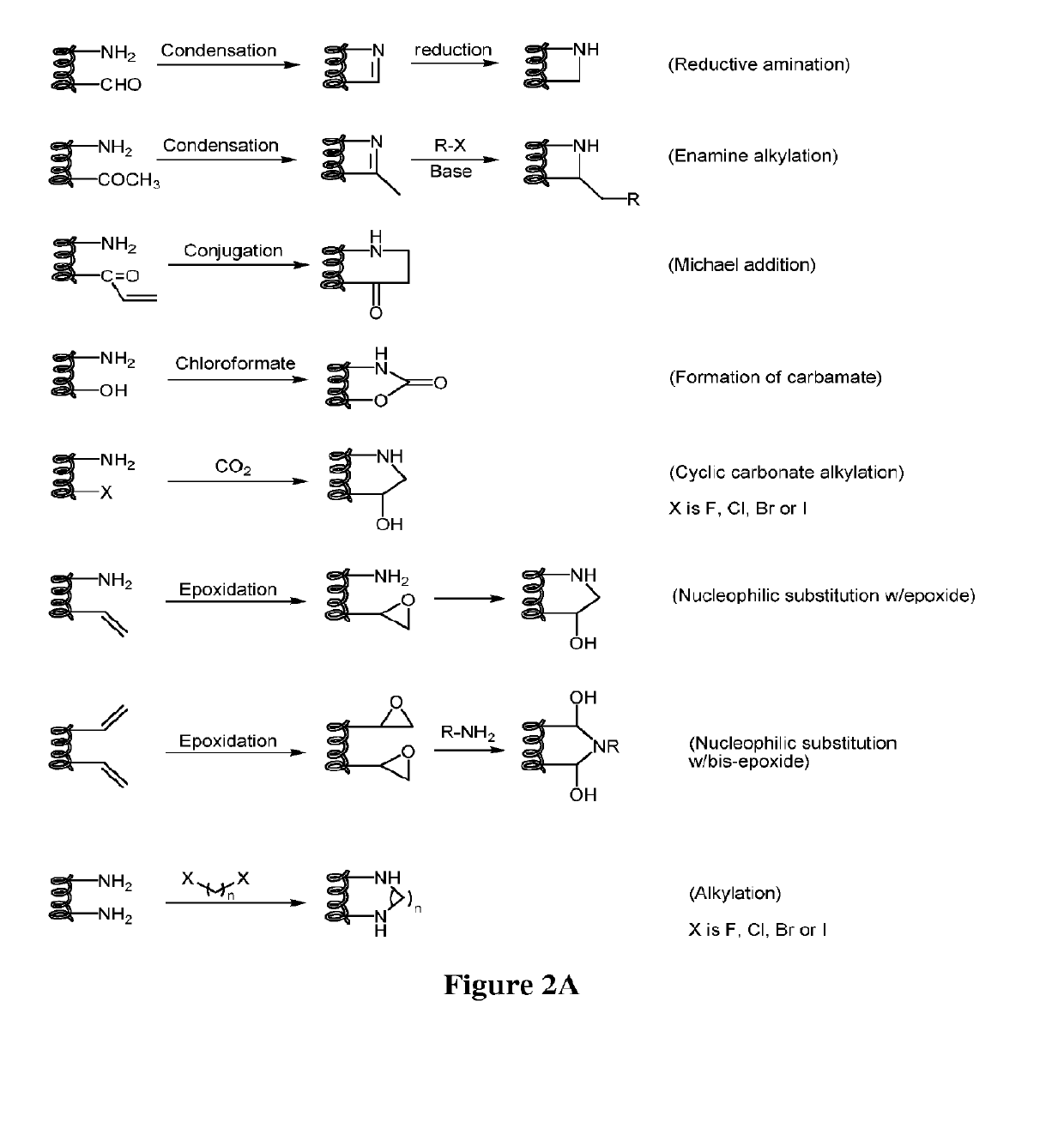
The invention relates to improvements in drug delivery and to the use of Cell Penetrating Agents (CPA's) or Cell Penetrating Peptides (CPP's) which have been stabilized by, for example: i) stapling two amino acids to form Stapled CPP's (StaP's) or ii) stitching three or more amino acids to form stitched CPP's (StiP's). More particularly there is provided a drug carrying cell penetrating molecule (DCCPM) comprising: a biologically active compound (BAC), and a cell penetrating agent (CPA), which BAC and CPA are linked directly or via a bi-functional linker (BFL). The CPA is a stabilized peptide(CPP) which has a conformation imposed upon it by stapling to form a stapled peptide (StaP) or stitching to form a stitched peptide (StiP). The StiP or StaP comprise a cross link or bridge between atleast two amino acids of the peptide and the cross link or bridge provides a cyclisation between at least two amino acids which are not formed by an olefin metathesis. Cyclisation may be achieved by one or more of: condensation of an aldehyde or ketone with a hydrazine or protected hydrazine; a thiol-ene Michael addition; a di-sulfide formation; a Huisgen 1, 3 di-polar cycloaddition; a reaction between an amine and carboxylic acid; a singlet or triplet based carbine reaction; or a Suzuki or Sonogashira coupling.
Owner:SUTURA THERAPEUTICS
Peptide derivative and pharmaceutical composition containing same
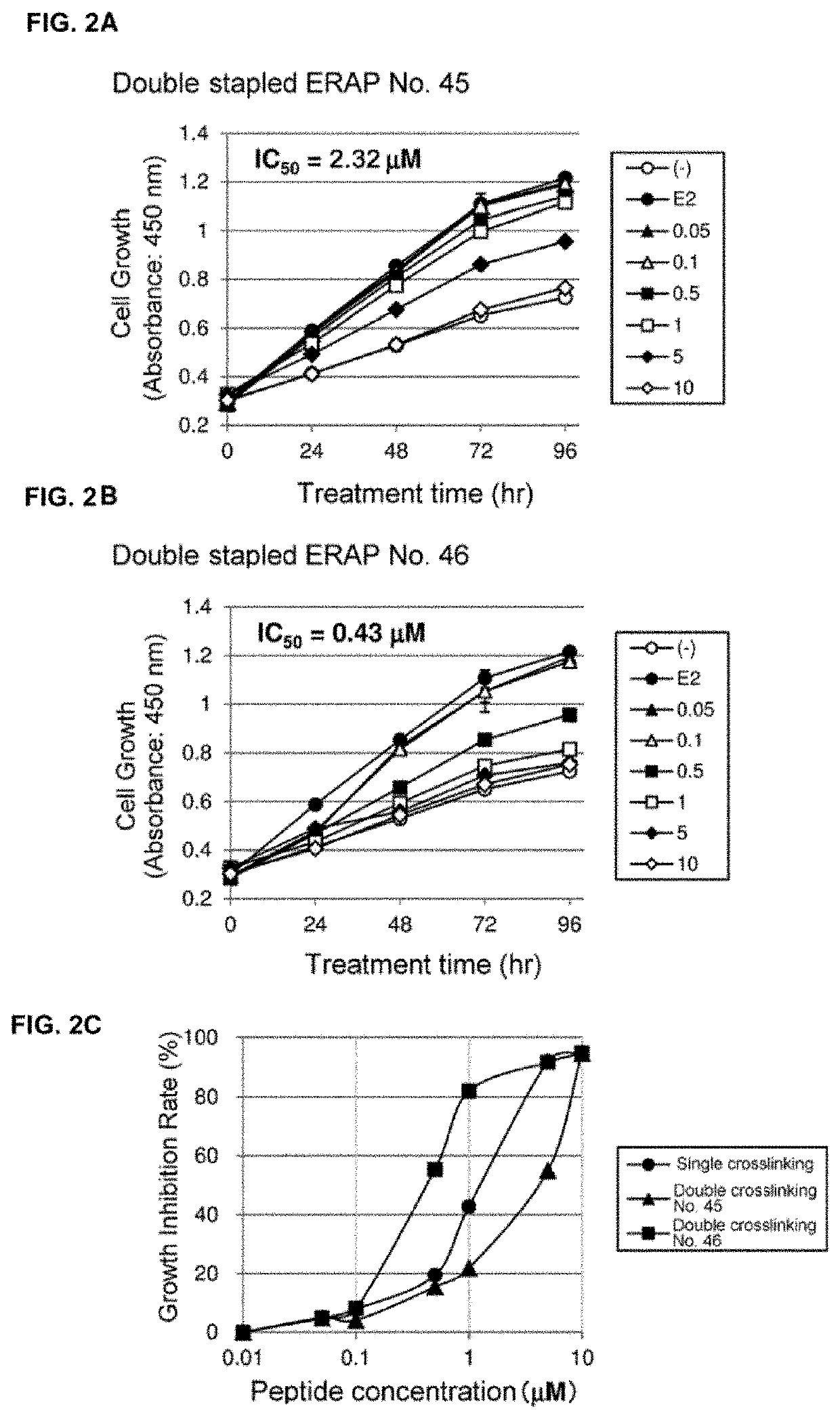
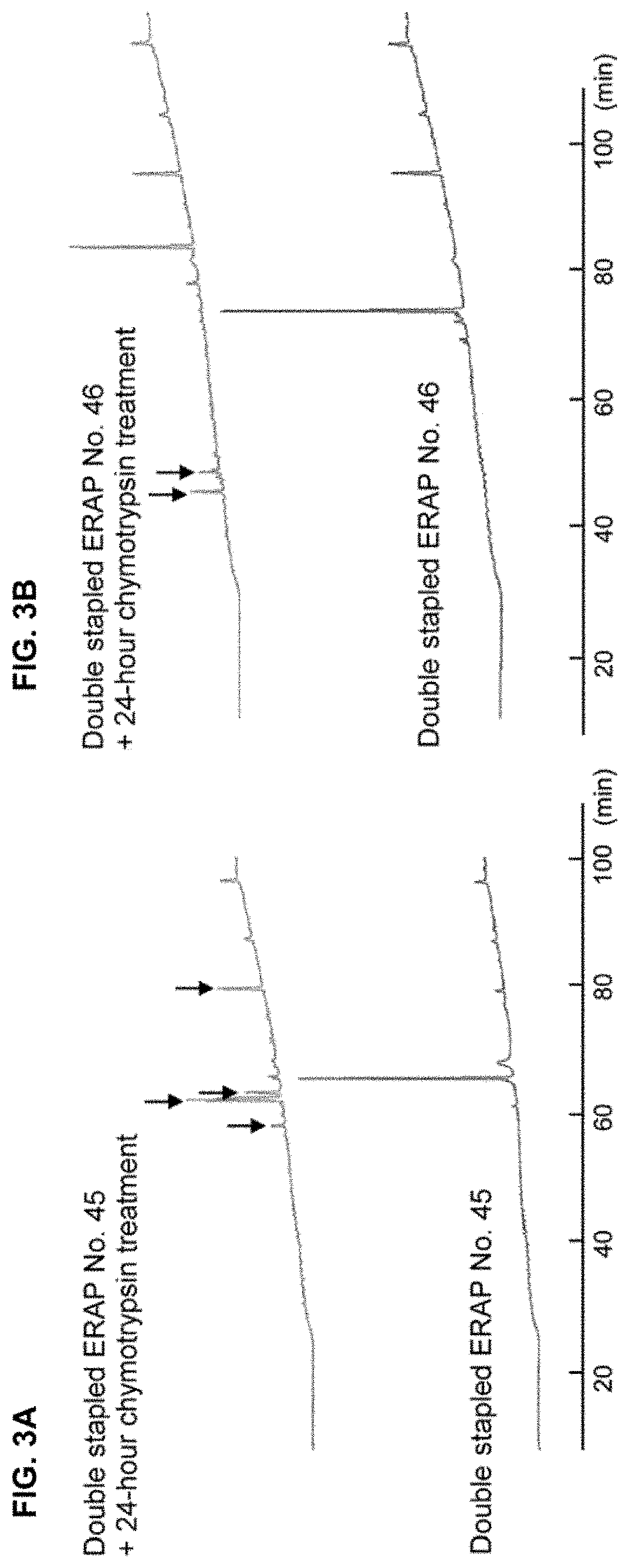
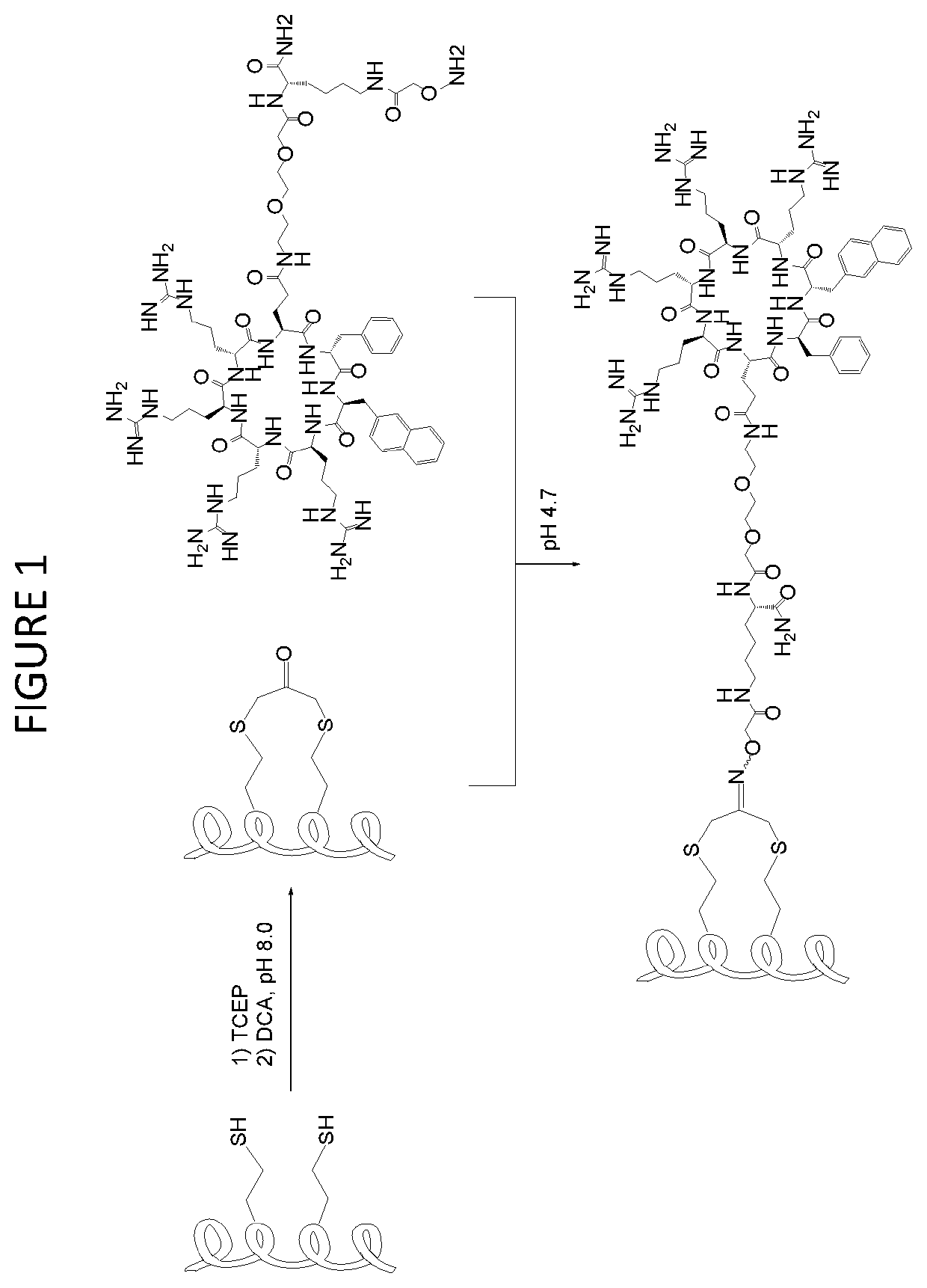
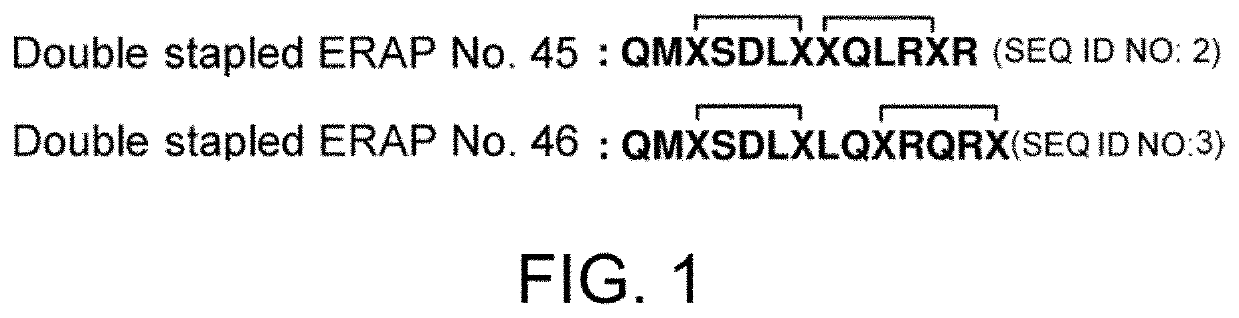
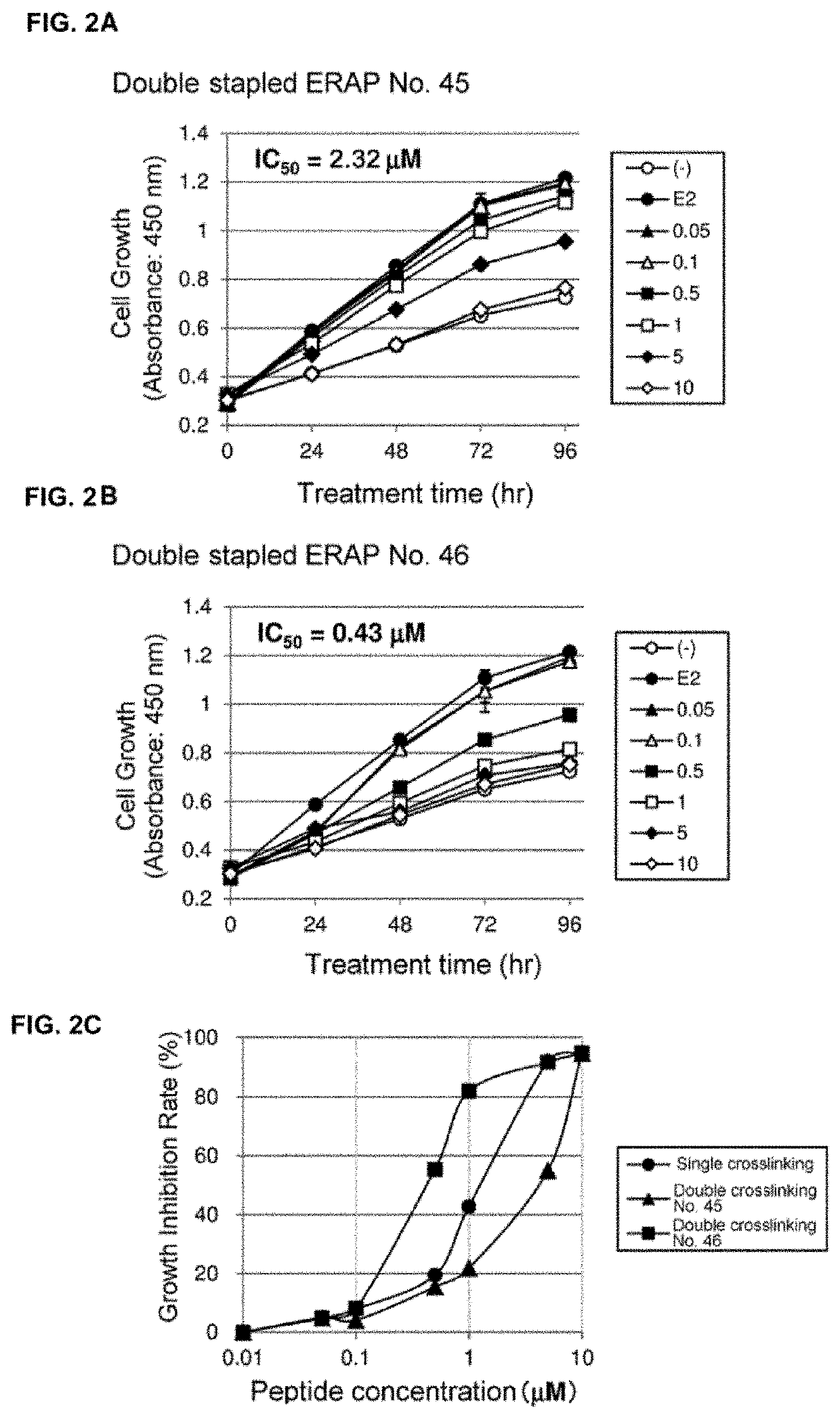
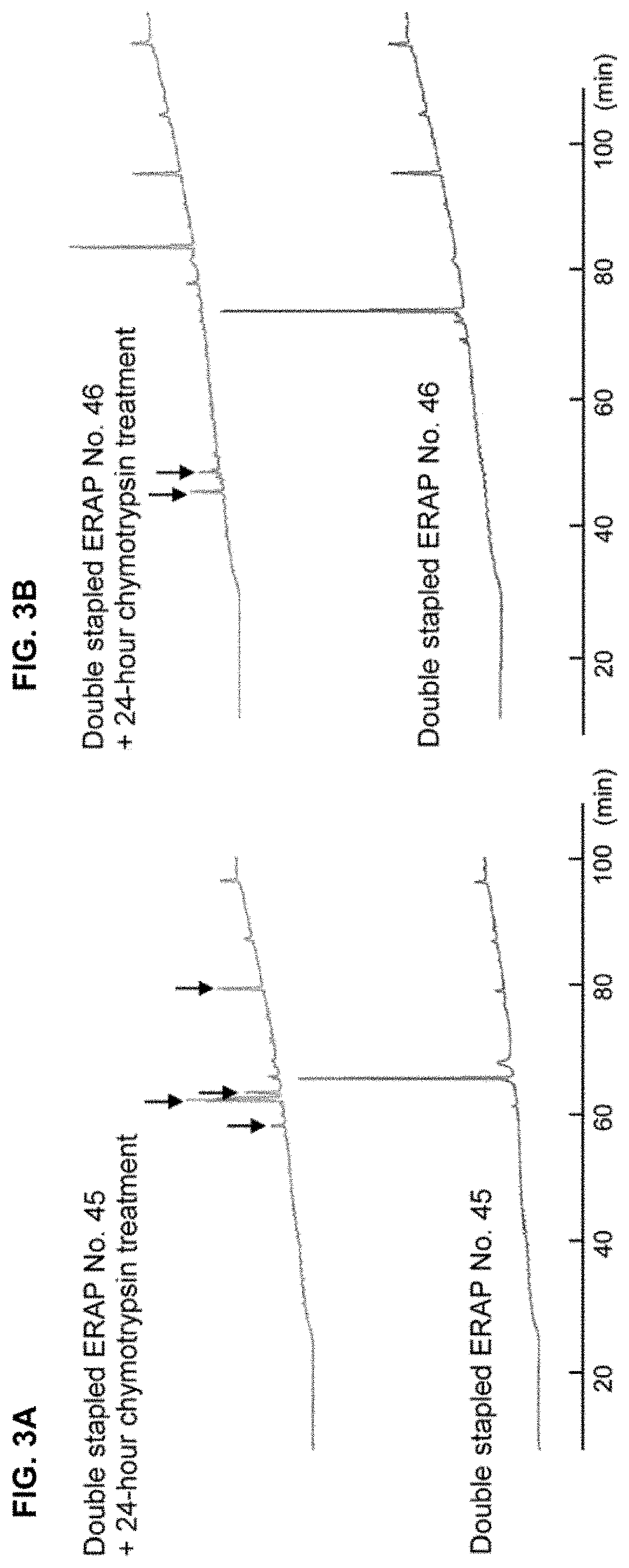
The present invention provides peptides having a structure in which portions of a dominant-negative peptide of BIG3 which inhibits the interaction between BIG3 and PHB2 are replaced by at least two stapling structures. The peptides of the present invention have excellent cell growth inhibiting activity. The cell growth inhibiting activity lasts longer, compared to a single-stapled peptide. Therefore, the peptides of the present invention have a feature suitable for clinical applications in cancer therapy.
Owner:ONCOTHERAPY SCI INC +1
Stapled beta-catenin ligands
PendingUS20220315631A1Superior cytosolic delivery efficiencyImprove bioavailabilityPeptide/protein ingredientsPharmaceutical non-active ingredientsStapled peptideCatenin Proteins
The present disclosure provides novel polypeptide conjugates. The polypeptide conjugates disclosed herein comprise a stapled peptidyl beta-catenin ligand and at least one staple which holds the peptidyl ligand in an α-helical confirmation, and a cell-penetrating peptide (CPP) conjugated, directly or indirectly, to the stapled peptide.
Owner:OHIO STATE INNOVATION FOUND
A stapled peptide that inhibits osteoclast differentiation and its preparation method and application
ActiveCN111233977BInhibition of differentiationHigh purityPeptide/protein ingredientsSkeletal disorderOsteoclastic differentiationStapled peptide
The present invention relates to a staple peptide for inhibiting osteoclast differentiation, a preparation method and application thereof. The present invention takes amino resin as carrier, and follows template FRATtide: Ac-DPHRLLQQLVLSGNLIKEAVRRLHSR-NH 2 The amino acid sequence was synthesized in the DIC-Oxime condensation system by Fmoc solid-phase synthesis method, and the peptide chain was synthesized on the basis of retaining key amino acid residues. 5 In place of the original amino acid, the linear peptide linked to the resin is subjected to olefin metathesis reaction and cyclization in the dichloroethane solution of Grubbs I reagent, and then cleaved from the resin to obtain the target stapled peptide. The method of the invention is simple and feasible, has high purity and high yield. Further experiments confirmed that the stapled peptide of the present invention can significantly inhibit the differentiation of osteoclasts, and has potential application value in the treatment of osteoporosis and other related diseases.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
Peptide derivative and pharmaceutical composition containing same
ActiveUS20200255474A1Longer lasting inhibitory effectPeptide/protein ingredientsPeptidesPharmaceutical drugStapled peptide
The present invention provides peptides having a structure in which portions of a dominant-negative peptide of BIG3 which inhibits the interaction between BIG3 and PHB2 are replaced by at least two stapling structures. The peptides of the present invention have excellent cell growth inhibiting activity. The cell growth inhibiting activity lasts longer, compared to a single-stapled peptide. Therefore, the peptides of the present invention have a feature suitable for clinical applications in cancer therapy.
Owner:ONCOTHERAPY SCI INC +1
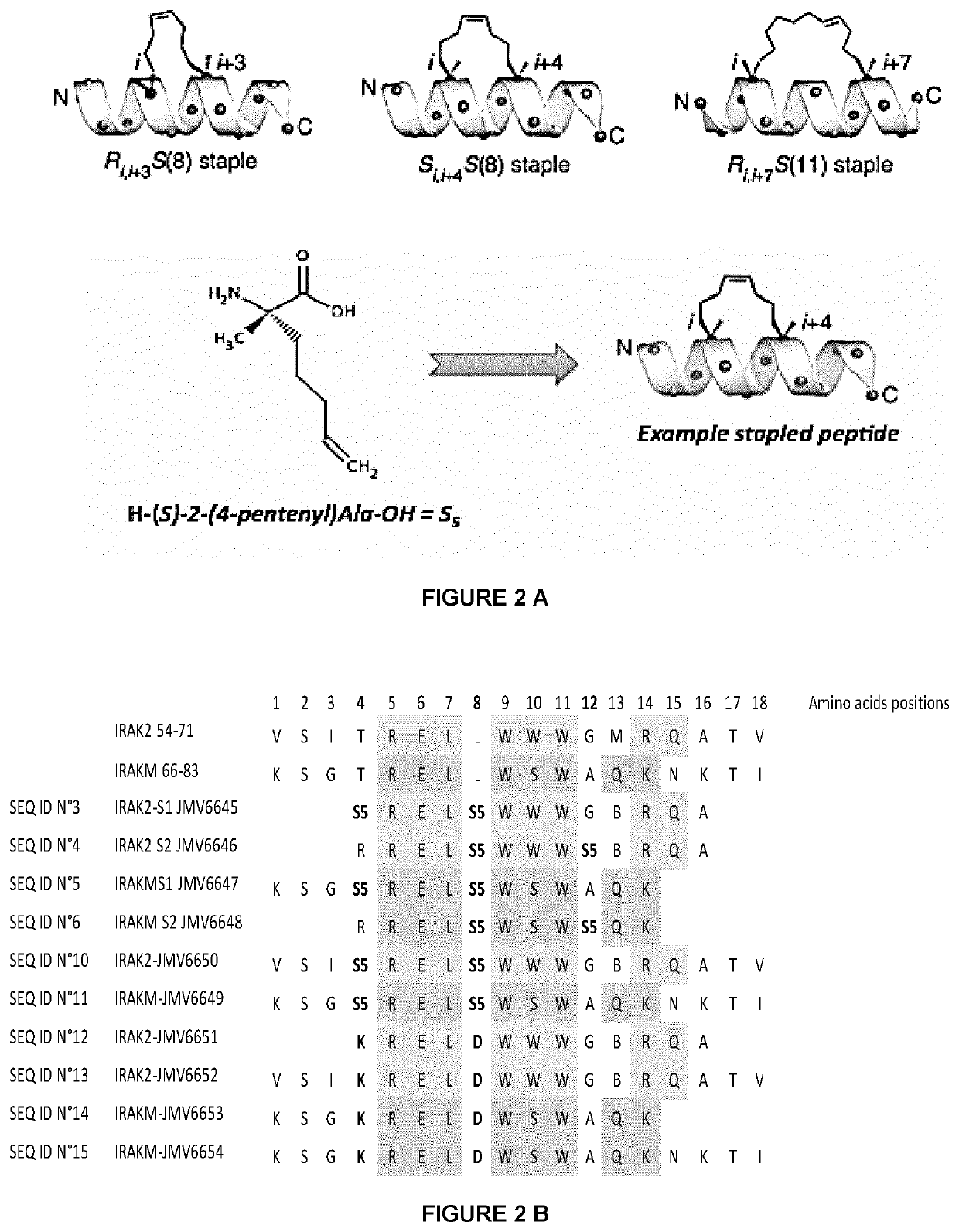
New stapled peptides and uses thereof
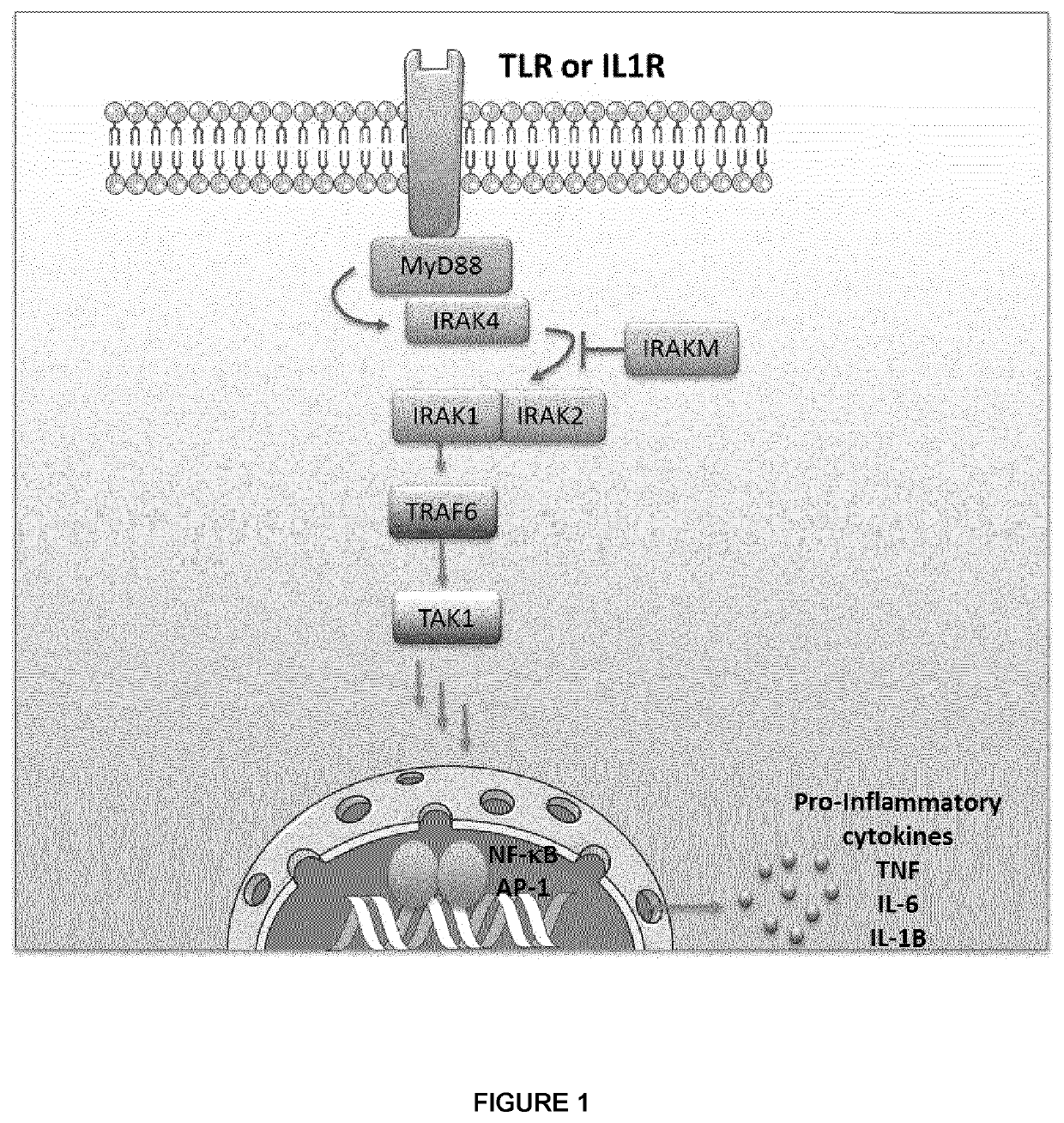
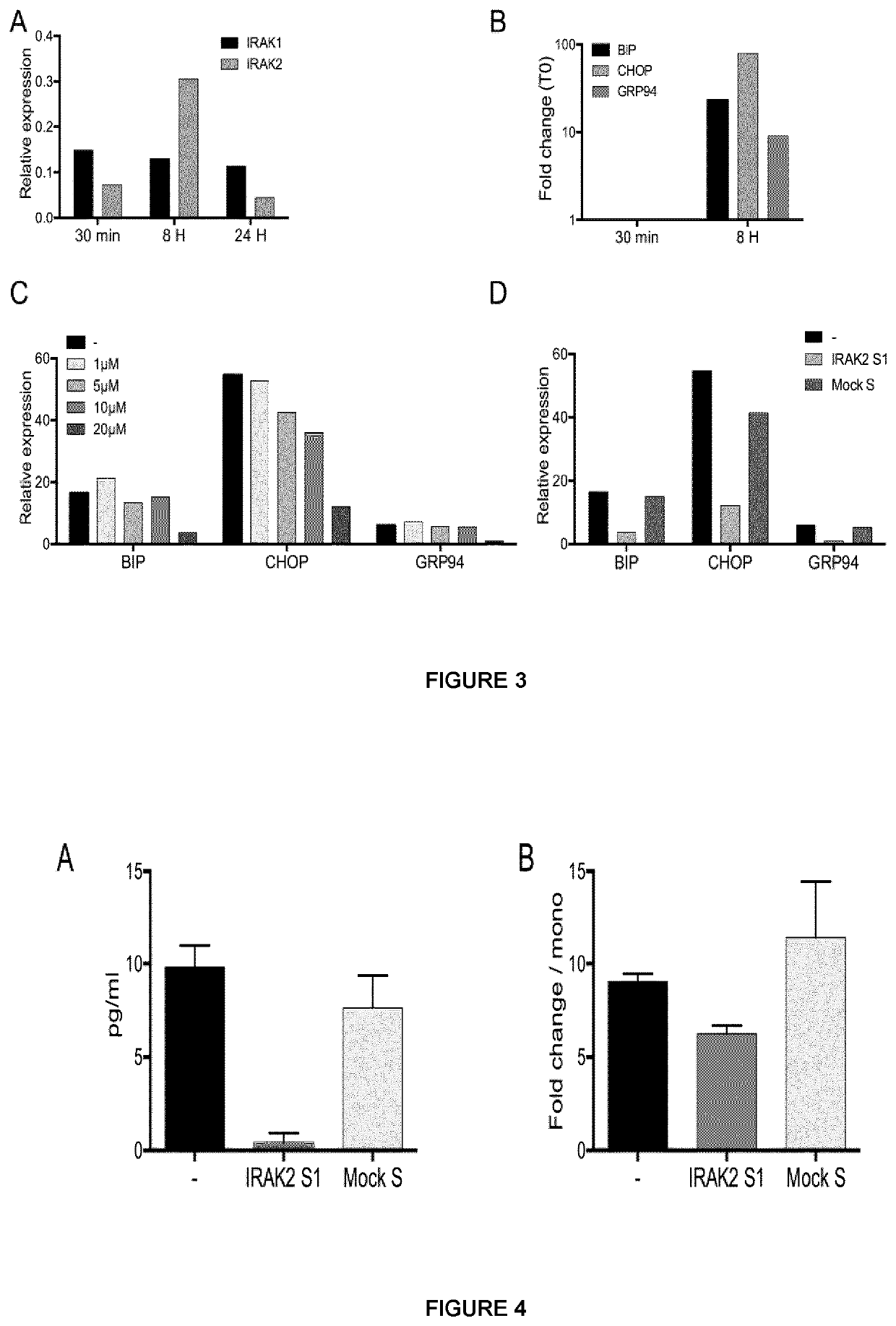
The present invention relates to peptidomimetic macrocycles comprising at least one macrocycle-forming linker and an amino acid sequence chosen from the group consisting of: i) an amino acid sequence with at least about 50%, 60%, 70%, 80%, 90%, or 95% sequence identity to a human sequence IRAK2 54-71 (SEQ ID No 1) and 100% identity with the amino acids in the positions 5-6, 9-11, 14-15 or ii) an amino acid sequence with at least about 50%, 60%, 70, 80%, 90%, or 95% sequence identity to a human sequence IRAKM 66-83 (SEQ ID No2) and 100% identity with the amino acids in the positions 5-6, 9-11, 13-14, wherein the peptidomimetic macrocycle comprises an α-helix and at least two natural or two non-natural amino acids crosslinked by a macrocycle-forming linker. It also concerns method of preparation of said peptidomimetic macrocycles and uses thereof, pharmaceutical composition and uses thereof, in particular as inhibitors of inflammatory pathways.
Owner:UNIV DE MONTPELLIER +4
Modulation of hepatitis B virus replication
Presently disclosed is a method of modulating Hepatitis B virus (HBV) replication, by contacting the cell with at least one agent that modulates at least one factor from a specified group consisting of SNAI2, SOX7 and other factors, the screening of said agent and use thereof in a medicament for treating HBV infection or disease or condition associated with a HBV infection in a subject. In one preferred embodiment, the agent is one peptide derived from SOX7 or SNAI2 or stapled peptides thereof. As a separate invention, a method of identifying at least one factor that modulates replication of a virus is also disclosed.
Owner:AGENCY FOR SCI TECH & RES
Hydrocarbon-stapled polypeptides for enhancement of endosome-lysosomal degradation
ActiveUS20180208627A1High trafficExcessive degradationPeptidesAntineoplastic agentsAutophagic deathPhysical interaction
The present invention relates to a Beclin 1-UVRAG complex structure which reveals a tightly packed coiled coil assembly with Beclin 1 and UVRAG residues complementing each other to form a stable dimeric complex. This potent physical interaction is critical for UVRAG-dependent EGFR degradation but less critical for autophagy. Targeting the Beclin 1 coiled coil domain with rationally designed stapled peptides leads to enhanced autophagy activity and EGFR degradation in non-small cell lung cancer (NSCLC) cell lines, suggesting translational value for these compounds.
Owner:THE HONG KONG POLYTECHNIC UNIV
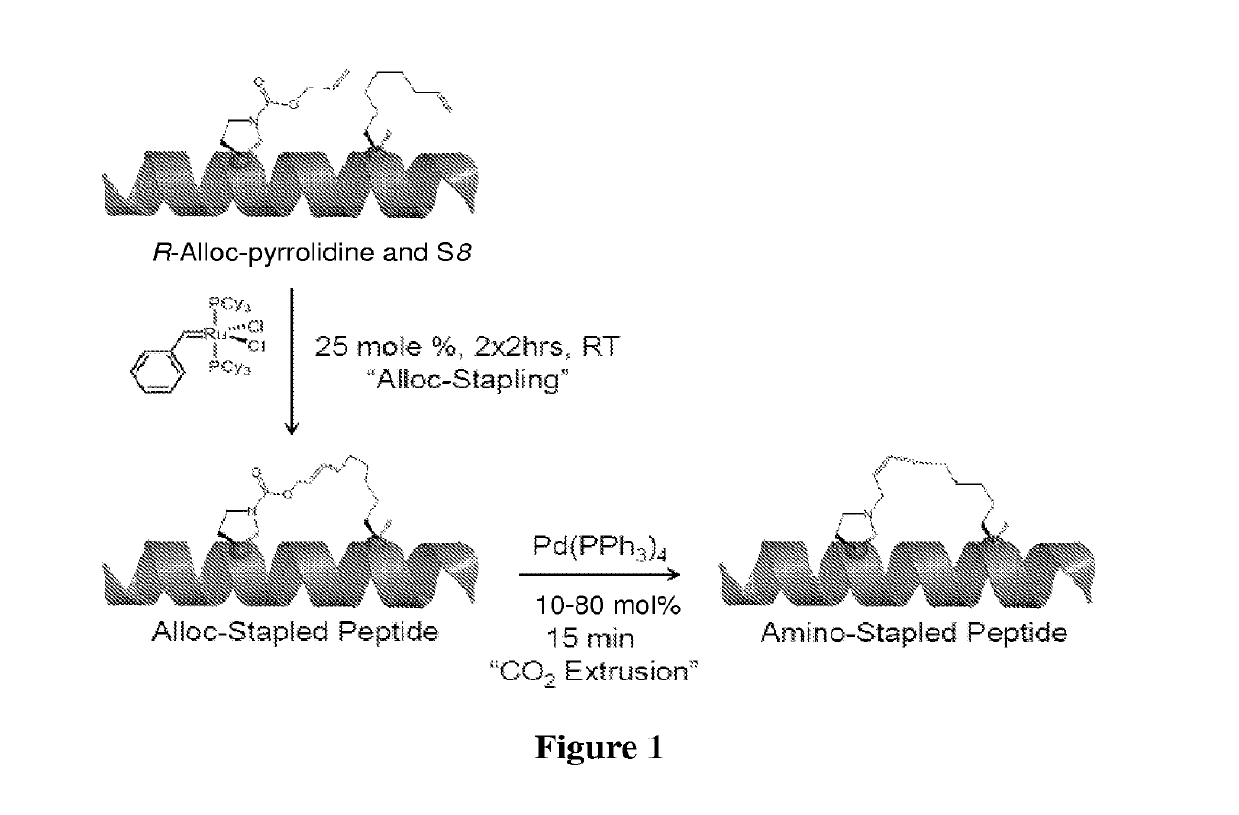
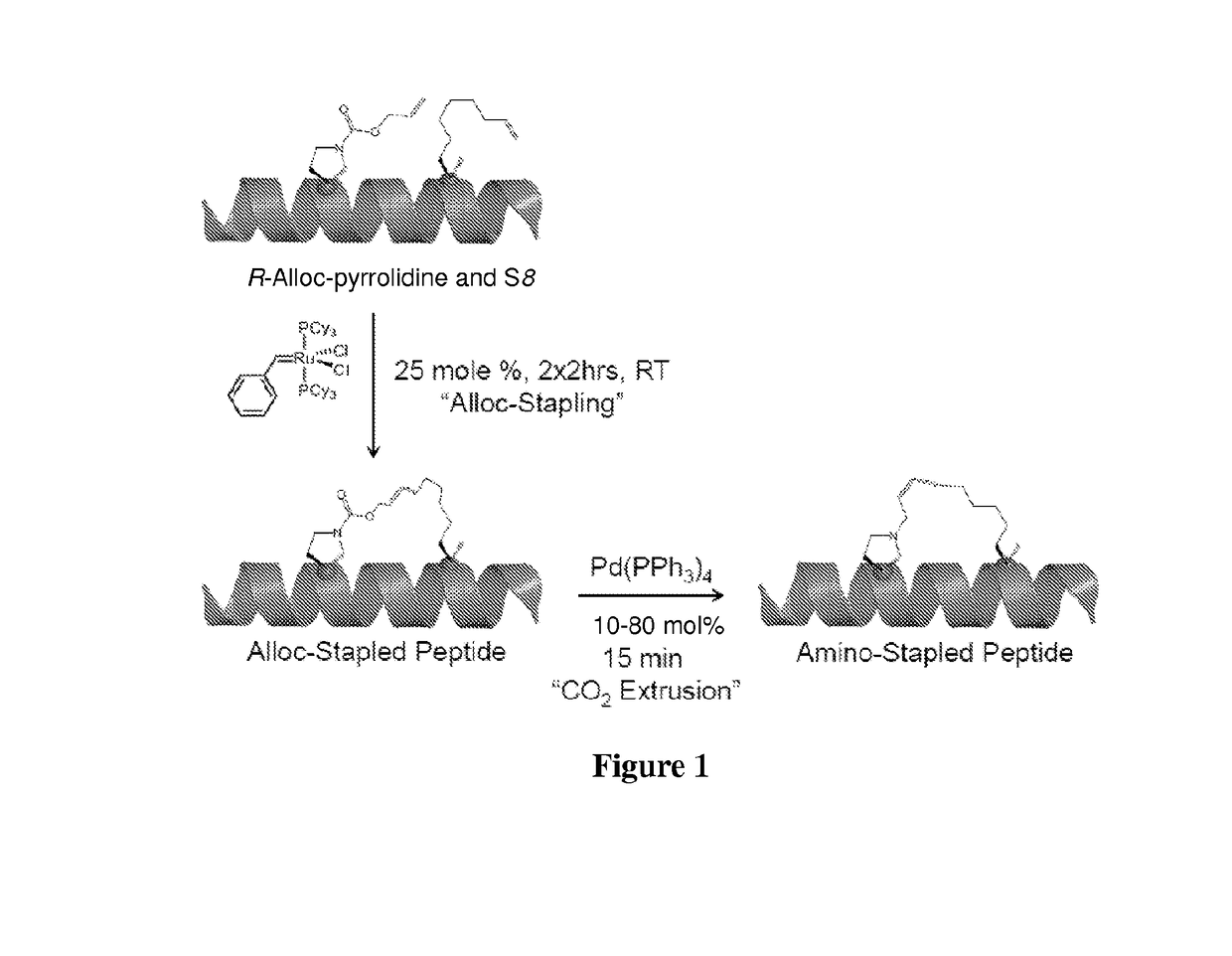
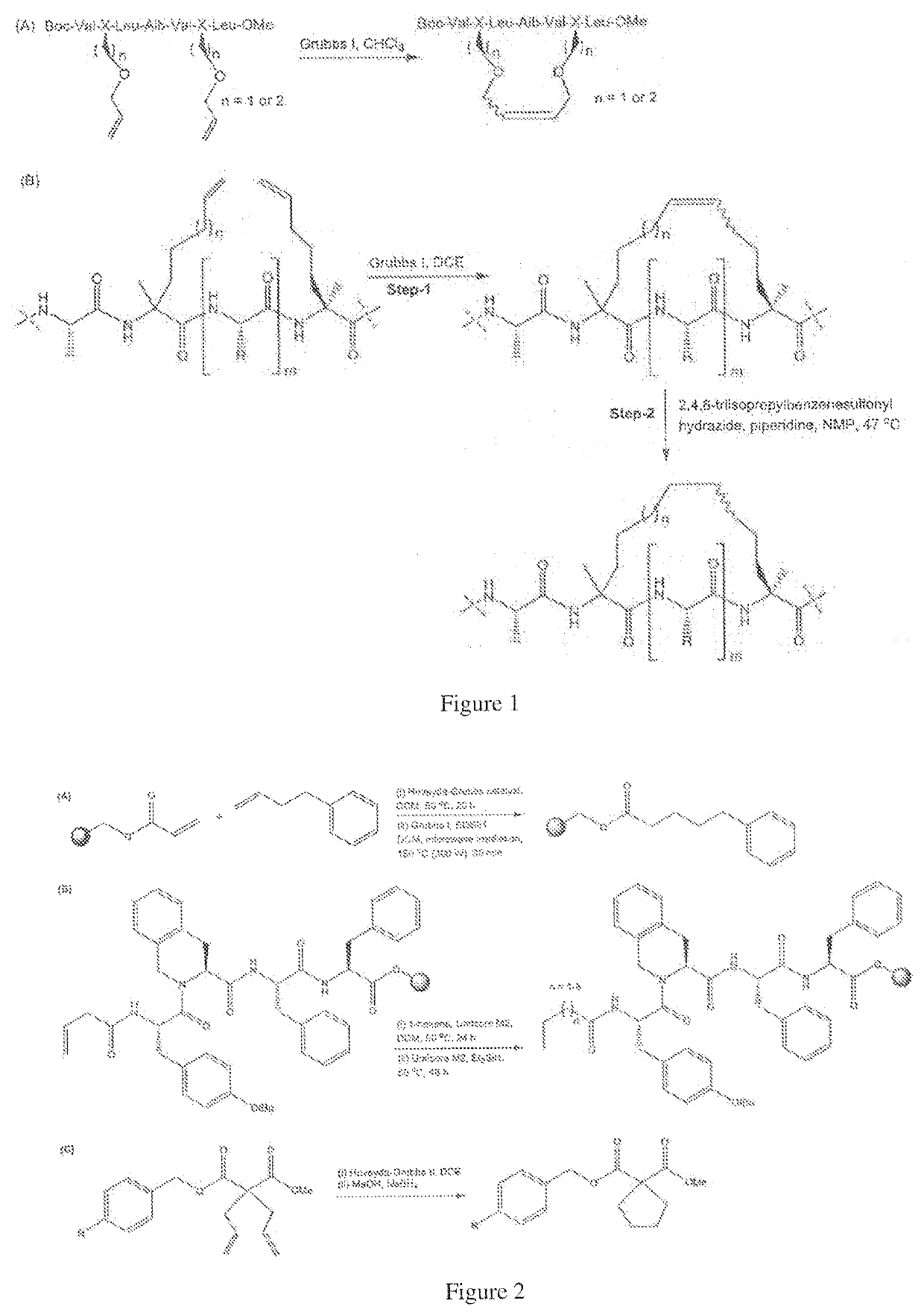
Method of preparing stapled peptides
ActiveUS11236128B2Ruthenium/rhodium/palladium/osmium/iridium/platinum compoundsPeptide preparation methodsCombinatorial chemistryStapled peptide
Described herein is an operationally simple, one-pot solid-supported preparation of saturated stapled peptides. Following completion of ruthenium-catalysed metathesis, solid-phase transfer hydrogenation was achieved using triethylhydrosilane at elevated temperatures. The utility of the method has been demonstrated on 14- and 16-mer peptides to yield the corresponding cyclic a-helix stabilised stapled peptides.
Owner:AGENCY FOR SCI TECH & RES
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com