Polypeptide conjugates for intracellular delivery of stapled peptides
A conjugate and conjugation technology, applied in the field of polypeptide conjugates, can solve problems such as poor cell membrane permeability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
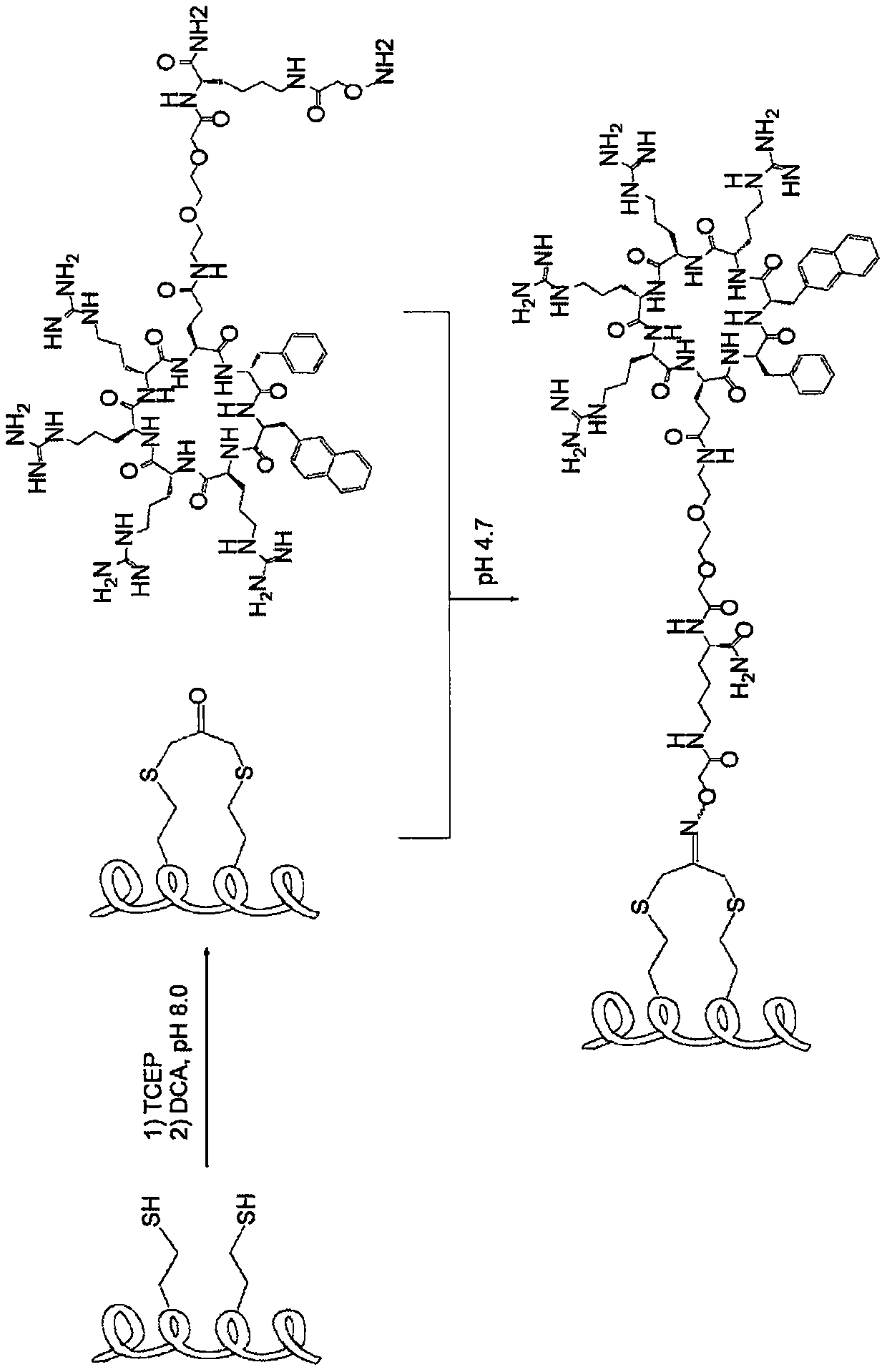
[0246] Example 1: Design strategy and synthesis of cyclic CPP binding peptide conjugate
[0247] We chose to prepare cCPP binding peptide conjugates by using a pooled synthesis method (Figure 1). First, the cargo peptide was synthesized by standard solid phase peptide synthesis (SPPS), in which two homocysteine residues were incorporated at positions i and i+4. After cleavage from the resin and side chain deprotection, the peptide was treated with 1.5 equivalents of 1,3-dichloroacetone (DCA) to bind the peptide into an α-helical conformation. The binding process also incorporates ketone groups into the binding peptide for subsequent bioorthogonal conjugation with cCPP. Next, cCPP [e.g., CPP9] is synthesized by SPPS using a miniature PEG-Lys (Mtt) linker attached to the side chain of Gln. While still on the resin, the Mtt group on the side chain of Lys was selectively removed by treatment with 5% trifluoroacetic acid (TFA), and the exposed amine was partially acylated with Boc...
example 2
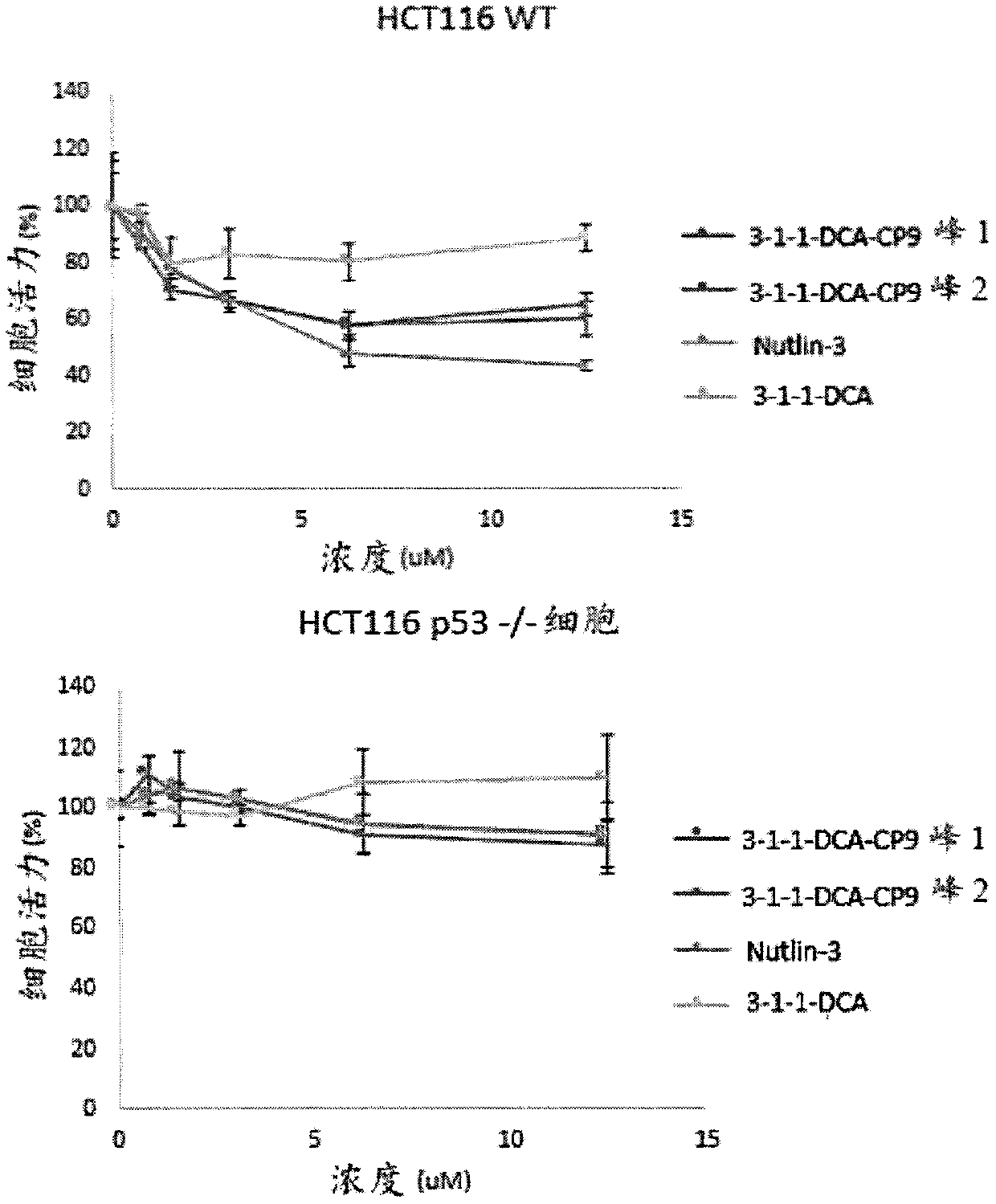
[0248] Example 2: Cell-permeable binding peptides against MDM2-p53 interaction
[0249] As a proof of concept, we synthesized a cell-permeable binding peptide that is resistant to MDM2-p53 interaction. The activation of the p53 protein can protect the organism from the proliferation of cells carrying damaged DNA with potentially carcinogenic mutations. MDM2 is a p53-specific E3 ubiquitin ligase and the main cell antagonist of p53, which acts to limit the growth inhibitory function of p53 in cancer cells. MDM2 mediates p53 monoubiquitination and proteasomal degradation. The use of small molecules and binding peptides to disrupt the p53-MDM2 complex has become a popular method of treating cancer with WT p53 protein. See Wade, M. et al., Nature Reviews Cancer 13, 83-96 (2013).
[0250] We selected the previously reported MDM2 ligand Ac-LTFEHYWAQLTS (SEQ ID NO:1) ("PDI"; see Phan, J. et al., J. Biol. Chem. 285, 2174-2183 (2010)), and passed The mini PEG-Lys linker is labeled with f...
example 3
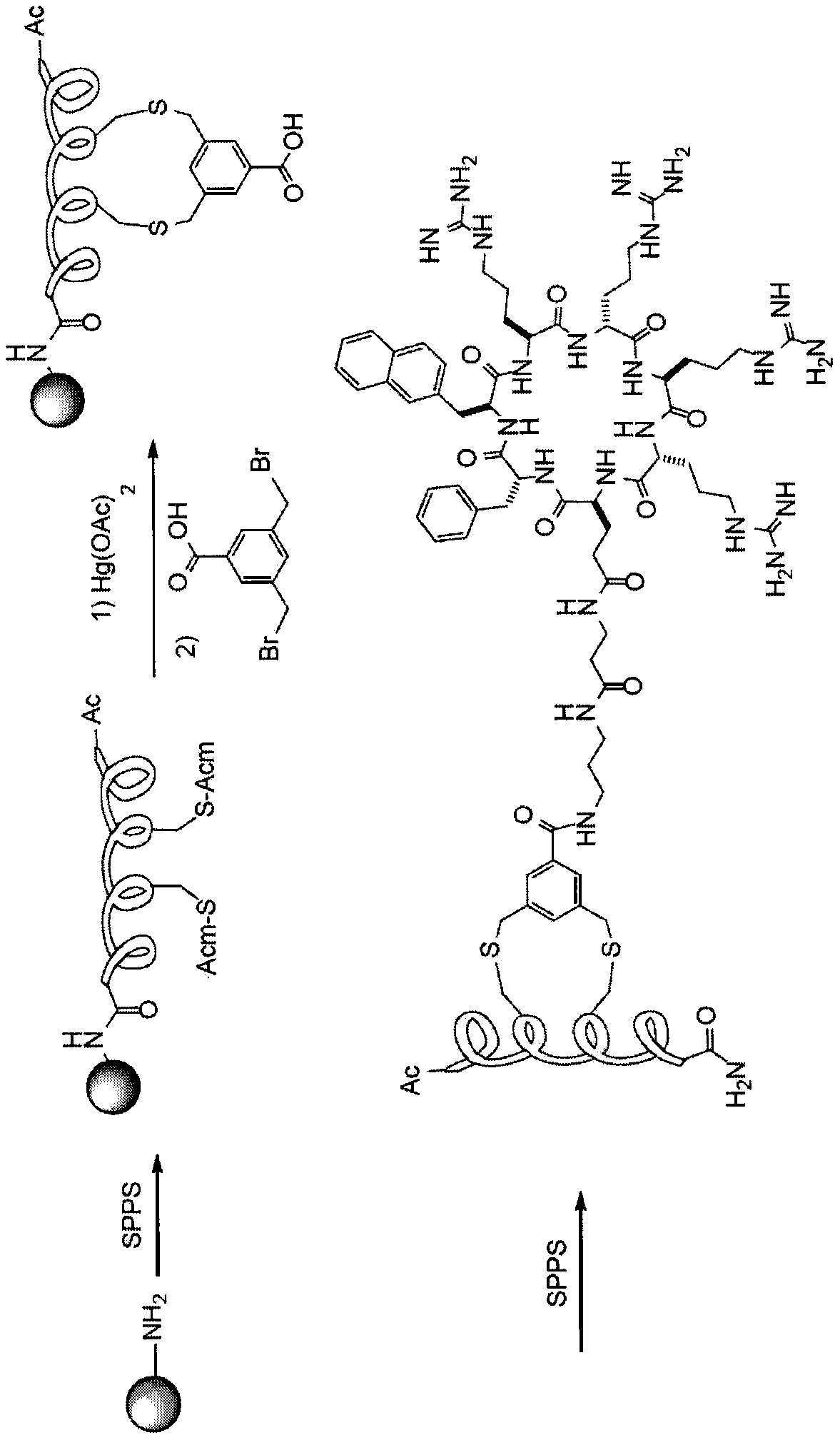
[0256] Example 3: Peptide binding and conjugation with 3,5-bis(bromomethyl)benzoic acid
[0257] The main limitation of oxime-based conjugation methods is the formation of two different stereoisomers, which can complicate product separation and further clinical development. To overcome this limitation, we next adopted 3,5-bis(bromomethyl)benzoic acid ("BBA") as the binding agent. The structurally similar compound meta-xylene dibromide has previously been used to bind α-helical peptides. See Jo, H. et al., J Am Chem Soc. 134, 17704-17713 (2012). The m-xylene dibromide reacts rapidly with two cysteines within spatial proximity, thereby forming a single bound peptide product with high yield and low reagent / peptide stoichiometry. We have developed two methods for binding / conjugating α-helical peptides to BBA. In the first method (Figure 3A), first, a cargo peptide containing two acetamidomethyl (Acm) protected cysteine is synthesized on a solid support by standard solid phase pe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com