Cationic bridged stapled peptide and application thereof
A staple and cationic technology, applied in the field of biomedicine, to achieve strong resistance to serum protease hydrolysis, reduce the effect of polypeptide biological activity, and long retention time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
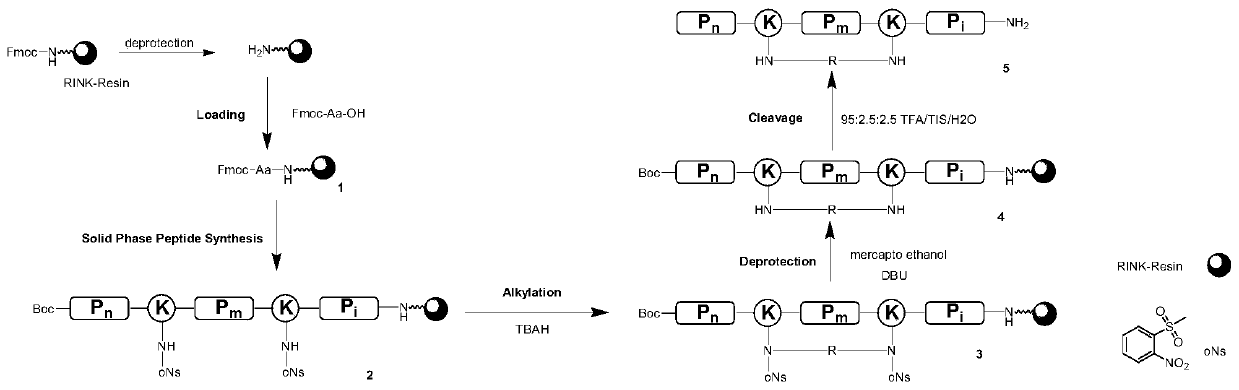
[0039] DBU: 1,8-diazabicyclo[5.4.0]undec-7-ene Example 1: Preparation of staple antimicrobial peptide
[0040] The preparation route is as follows, such as figure 1 Shown:
[0041] 1. Preparation of oligopeptide solid-phase resin: Rink-AM resin (loading capacity is 0.35mmol / g, initial loading capacity of each peptide is 200mg), Fmoc-Val-OH is loaded on Rink-AM by standard solid-phase synthesis method AM resin.
[0042] 2. Preparation of lysine protected by o-nitrobenzenesulfonyl group in the side chain: Weigh 4.68g of Fmoc-Lys(Boc)-OH and place it in a 250mL round bottom flask, then measure the solvent CH 2 Cl 2 60mL and 20mL of trifluoroacetic acid (TFA) were added to the round bottom flask, and 10mL of CH 2 Cl 2 Rinse the measuring cylinder that has taken TFA, and transfer the rinsing solution to a round bottom flask, and stir for 1 h. To the obtained intermediate Fmoc-Lys-OH·TFA, add H 2 0: Tetrahydrofuran (THF) = 1:1 (55 mL each), N-ethyldiisopropylamine neutralize...
Embodiment 2
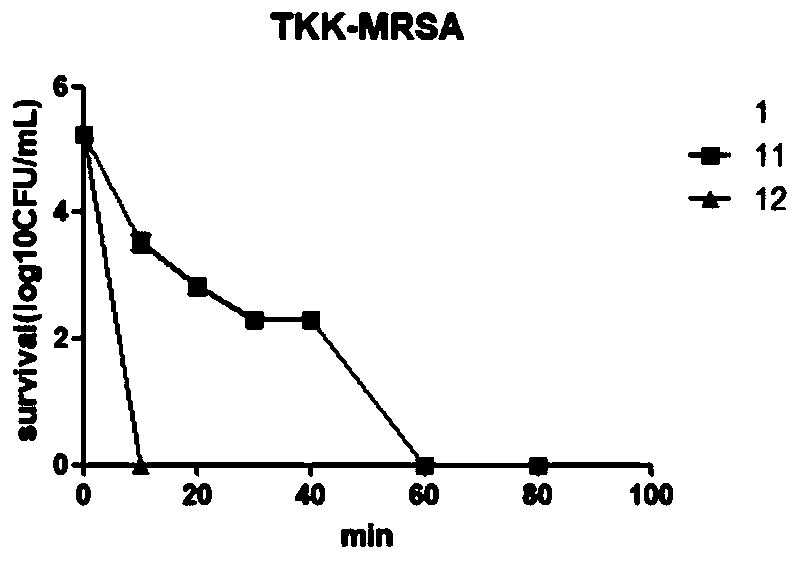
[0059] Embodiment 2: the effect of antimicrobial peptides of staples in inhibiting bacterial growth
[0060] The antibacterial activity detection was carried out according to the conventional doubling dilution method. Add 180uL of bacterial solution and 20uL of drug solution to each well of the first row of 96-well plate, (drug concentration 128ug / mL) set up 3 multiple wells in each well, use TSB medium as negative control, and then doubling dilution to make the final concentration double Decrease, incubate at 37 degrees for 16-20h, measure the absorbance of each well, the lowest concentration with no change in absorbance is the minimum inhibitory concentration (MIC).
[0061] Table 1. Effects of Staple Peptides on Bacterial Growth Inhibition
[0062]
[0063] It can be seen from Table 1 that the staple antimicrobial peptide has broad-spectrum and high-efficiency antibacterial activity, and the staple peptide compound 115 connected with 1,2-dimethylene phenyl is effective ...
Embodiment 3
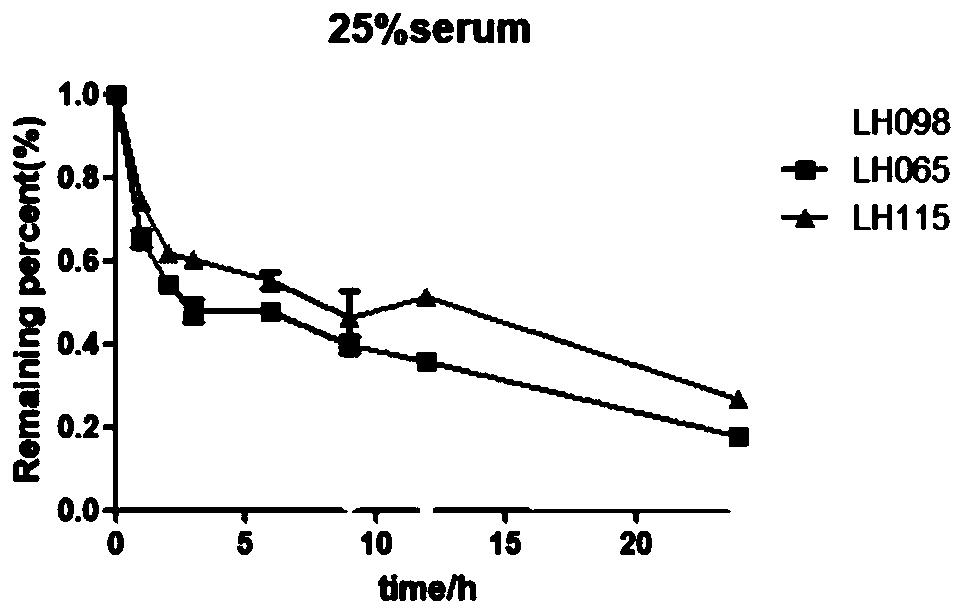
[0064] Example 3: Staple Antimicrobial Peptide Resistance to Serum Hydrolysis
[0065] Experimental method: the peptides (LH098, LH065, LH115) were incubated with 25% fresh human serum (v / v) at 37°C (the final concentration of the peptide was 1.28 mg / mL). Take 60ul at different time points (0, 1h, 2h, 3h, 6h, 9h, 12h and 24h), then add 120uL of 12% trichloroacetic acid solution (water: acetonitrile 1:3), precipitate serum at 4°C for 30min protein. Then centrifuge at 14000rpm for 10min, take the supernatant, and analyze it by high performance liquid chromatography. See the experimental results figure 2 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com