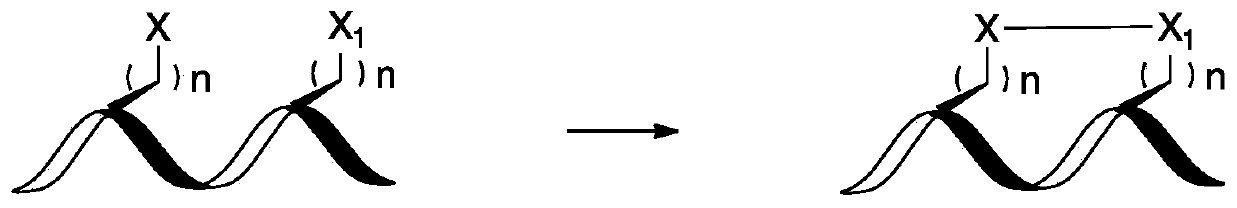
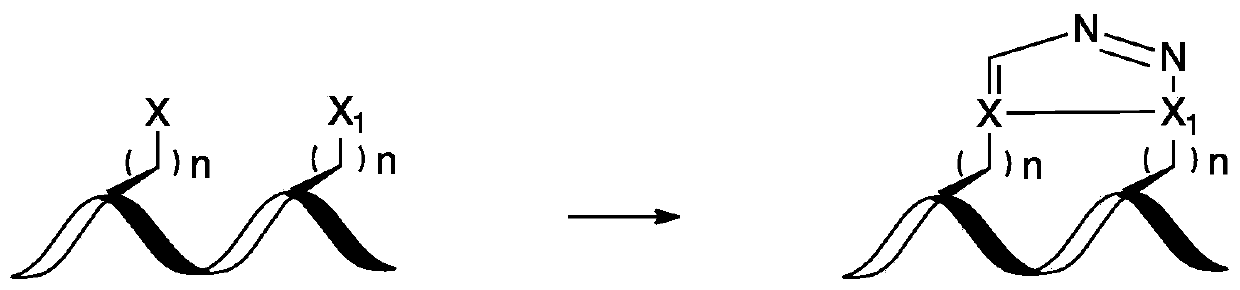
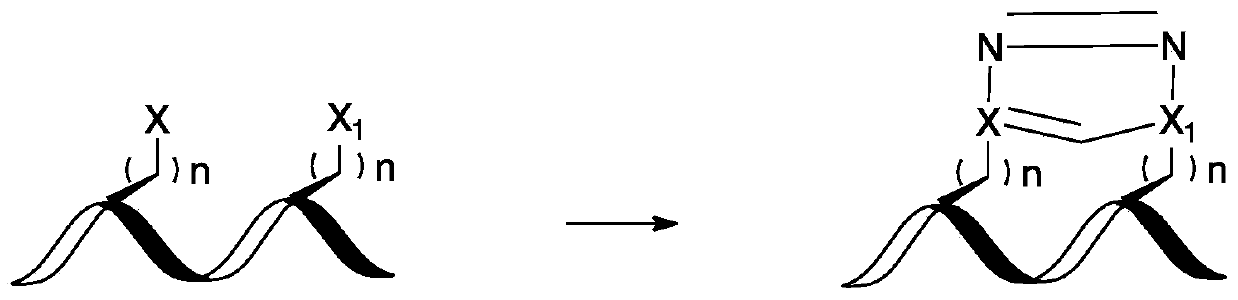
Compounds comprising stapled or stitched peptides for improved drug delivery
A compound, drug technology, applied in the field of drug delivery improvement, can solve the problems of structural instability, large membrane toxicity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example
[0248] Primary validation provided by PCT / GB2016 / 054028 with PMO CP8M and PMO HP8M as comparators against alternative chemicals described herein.
[0249] DCCPM enhances RNA steric blockade in the treatment of Duchenne muscular dystrophy (DMD).
[0250] Introduction
[0251] Duchenne muscular dystrophy (DMD) is the most common genetically fatal childhood disease in the world, affecting approximately 1 in 4,000 live births worldwide 37 . This severe muscle wasting disorder is caused in most families by mutations in the gene that result in breaks in the reading frame and premature truncation of dystrophin 38,39 .
[0252] RNA splicing inhibition of DMD transcripts holds particular promise. Hybridization of AO to specific RNA sequence motifs prevents the correct assembly of the spliceosome, thereby failing to recognize target exons in the pre-mRNA, thus excluding them from the mature gene transcript. AO-mediated inhibition of RNA splicing leads to re-expression of truncate...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com