Thioester stapled peptide, preparation method and application thereof
A technology for stapling peptides and thioesters, applied in the field of peptides, to avoid product isomerization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Example 1 Synthesis of Thioester Stapled Peptide
[0043] raw material
[0044] Buffer A: 6M Guanidine Hydrochloride, 100mM Na 2 HPO4 ,pH=8.5;
[0045] Buffer B: 6M Guanidine Hydrochloride, 100mM NaH 2 PO 4 ,pH=2.5;
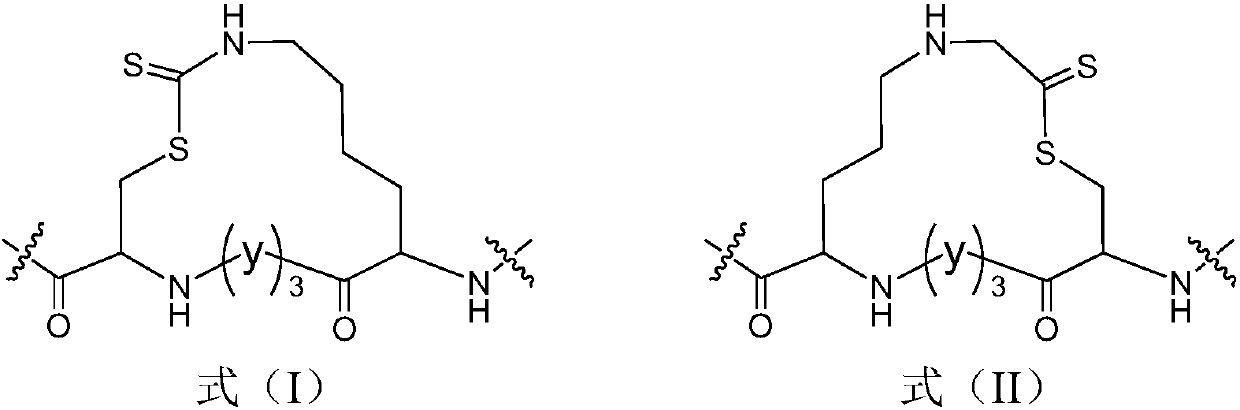
[0046] Table 3 Linear peptides
[0047]
[0048] The linear peptides shown in Table 3 can be prepared by solid-phase polypeptide synthesis (SPPS).
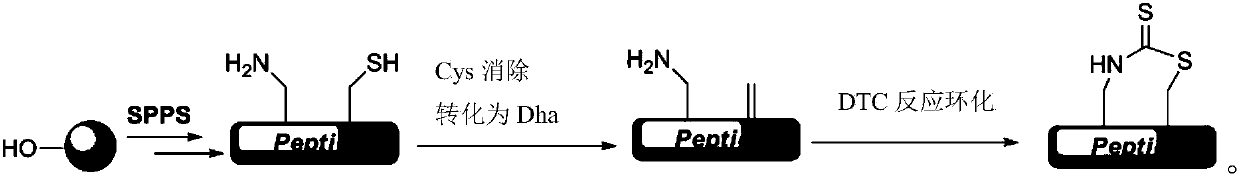
[0049] The preparation of thioester-stapled peptide by linear peptide PS1-1-1 is described below as an example, and the operation of preparing thioester-stapled peptide by other linear peptides is the same.
[0050] synthetic route:
[0051]
[0052] Synthetic steps:
[0053] (a) Cys is removed to Dha
[0054] Dissolve linear peptide PS1-1-1 (50 mg) in buffer B (3 mL), then slowly drop into buffer A (47 mL) dissolved with 2,5-dibromoadipamide (75 mg), at room temperature After stirring overnight, HPLC detected that the reaction was complete, purified by preparative liquid phase and lyophilized ...
Embodiment 2
[0060] Embodiment 2 Fluorescence polarization binding experiment
[0061] First, TAMRA-NHS (tetramethylrhodamine-N-hydroxysulfosuccinimide) was covalently linked to the N-terminal amino group of PMI (TSFAEYWNLLSP), and a fluorescent label with TAMRA as the fluorescent group was obtained. The peptide PMI-TAMRA was subsequently determined to have binding constants of 0.62 nM and 0.72 nM for PMI-TAMRA to MDM2 and MDMX, respectively. In order to verify the specificity of this method, the competitive binding constant K of the complex system of PMI-TAMRA and MDM2 or MDMX was determined without fluorescent labeling i . In addition, as a control, the competitive binding constant K of Nutlin-3 to MDM2 or MDMX was determined at the same time i , are 5.1 nM and 1.54 μM, respectively, which are in agreement with the reported K i The values are basically the same. K i The smaller it is, the higher the binding ability is, and the modified polypeptide PMI of the present invention has ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com