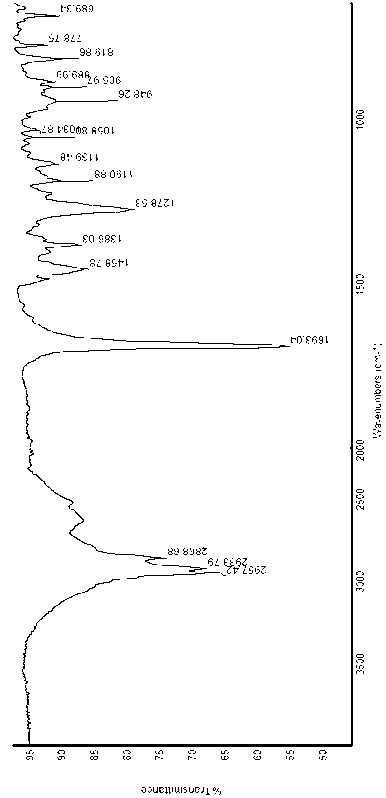
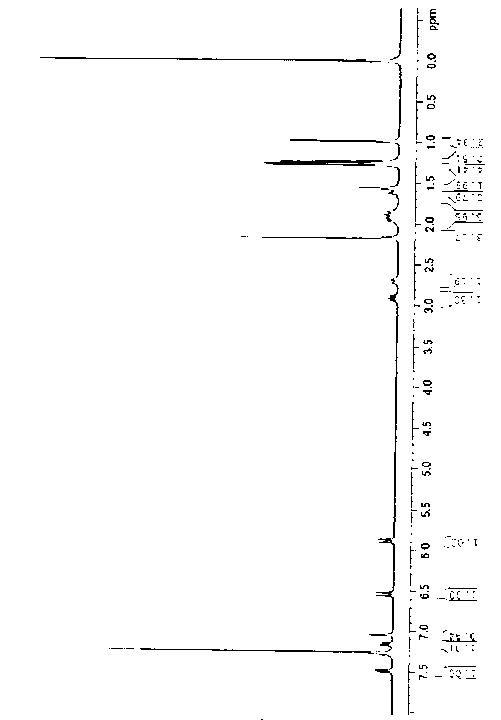
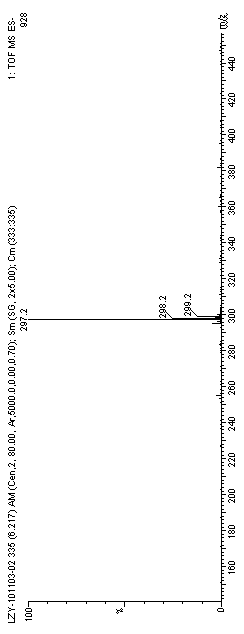
Preparation method of 6,8,11,13-tetra-abietic olefine acid
A technology of abietic acid and rosin, which is applied in the preparation of carboxylates, the preparation of organic compounds, chemical instruments and methods, etc., can solve the problems of no discovery, extraction, preparation, and difficulty in activity research, and achieve rapid reaction, avoidance of The effect of organic solvent purification and fewer by-products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] A kind of preparation method step of 6,8,11,13-abietonic acid is:
[0028] Step 1: Add 10 parts by mass of rosin to 5 to 100 parts by mass of an organic solvent with 2 to 6 carbons, stir evenly, and heat to completely dissolve the rosin; the organic solvent with 2 to 6 carbons used is dichloromethane, 1, Any one or more of 4-dioxane, tetrahydrofuran or acetone.
[0029] Step 2: Add the rosin solution dissolved in the previous step into a fixed-bed reactor equipped with an oxidizing agent and a dehydrating agent. The fixed-bed reactor maintains a temperature of 30-70°C and a reaction time of ≤120min. Obtain 6,8,11,13-abietonic acid solution; the oxidizing agent is any one of dichlorodicyanoquinone, selenium dioxide, and manganese dioxide; the dehydrating agent is 4A molecular sieve, 5A Molecular sieve, any one of p-toluenesulfonic acid.
[0030] Step 3: filter the 6,8,11,13-arbitronic acid solution in the above step, distill, recrystallize, and then vacuum dry to obtai...
Embodiment 2
[0032] Put 10 parts of rosin into 20 parts of dichloromethane solvent, heat to 50° C., and dissolve them all under stirring. Add the dissolved rosin solution into the fixed-bed reactor equipped with dichlorodicyanoquinone and 4A molecular sieve. The reaction was heated for 10 minutes to obtain a 6,8,11,13-arbitronic acid solution; the content of 6,8,11,13-arbitronic acid in the solution was analyzed by gas chromatography, and its content was 78%. The reaction solution was filtered, distilled, and recrystallized from acetone to obtain the target product, and the content of 6,8,11,13-abietonic acid was more than 95% according to liquid chromatography (HPLC).
Embodiment 3
[0034] Put 10 parts of rosin into 20 parts of 1,4-dioxane solvent, heat to 50° C., and dissolve it completely under stirring. Add the dissolved rosin to the MnO 2 and 4A molecular sieve in a fixed bed reactor. The reaction was heated for 20 minutes to obtain a 6,8,11,13-arbitronic acid solution; the content of 6,8,11,13-arbitronic acid in the solution was analyzed by gas chromatography, and its content was 80%. The reaction solution was filtered, distilled, and recrystallized from acetic acid to obtain the target product, and the content of 6,8,11,13-arbitronic acid was more than 97% according to liquid chromatography (HPLC).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com