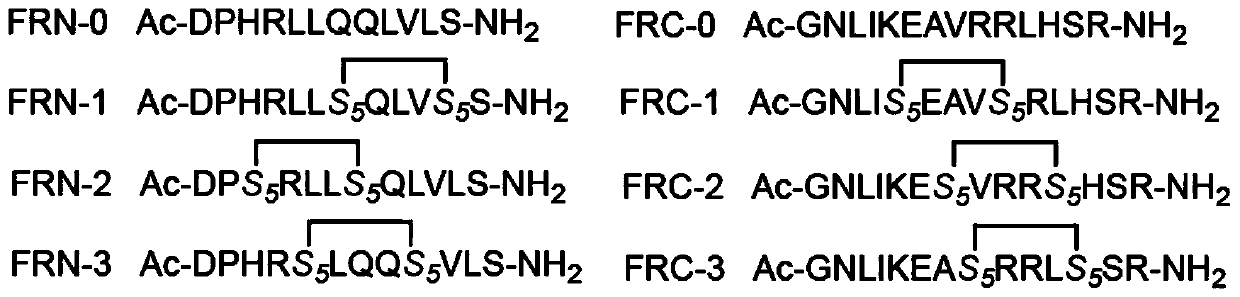
Stapled peptides for restraining osteoclast differentiation and preparation method and application of stapled peptides for restraining osteoclast differentiation
A technology for inhibiting osteoclasts and stapling peptides, applied in the field of polypeptide drugs, can solve problems such as the role of osteoclast differentiation not seen, and achieve the effect of high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0066] Example 1 Preparation of Staple Peptide Inhibiting Osteoclast Differentiation in the Present Invention
[0067] 1. Synthesis of stapled peptide
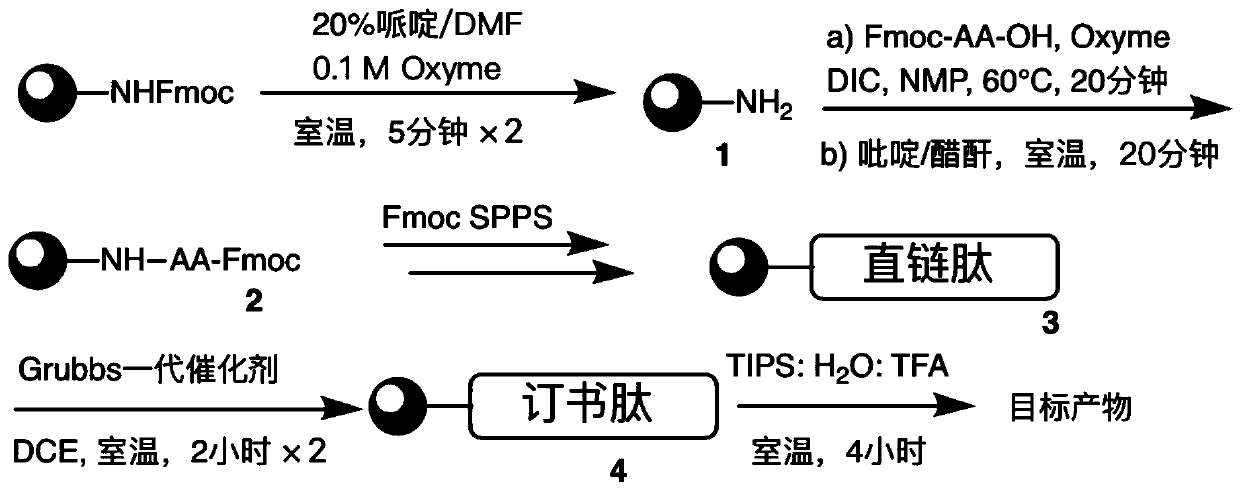
[0068] Such as figure 2 Shown:
[0069] (1) Preparation of compound 1
[0070] Take 500 mg of amino resin (sample loading is 0.30 mmol g -1 ) into the solid-phase synthesis reaction tube, soaked in DCM for 20 minutes to fully swell the resin, and drained for later use.
[0071] Add 20% piperidine-DMF solution (0.1M Oxyme) until the resin is completely submerged, shake at 25°C for 5 min×2 to remove Fmoc on the resin, and wash the resin with DCM and DMF for 3 times.
[0072] (2) Preparation of Compound 2
[0073] The first amino acid in the sequence (1mmol), Oxyme (142mg, 1mmol) and DIC (155.0μL, 1mmol) were mixed in 6ml NMP, added to the resin and shaken at 60°C for 20min (S 5 The last amino acid was reacted for 1 h, and the reaction was repeated once), and the resin was washed with DCM and DMF successively for 3 times e...
Embodiment 2
[0086] Identification and structure analysis of embodiment 2 product
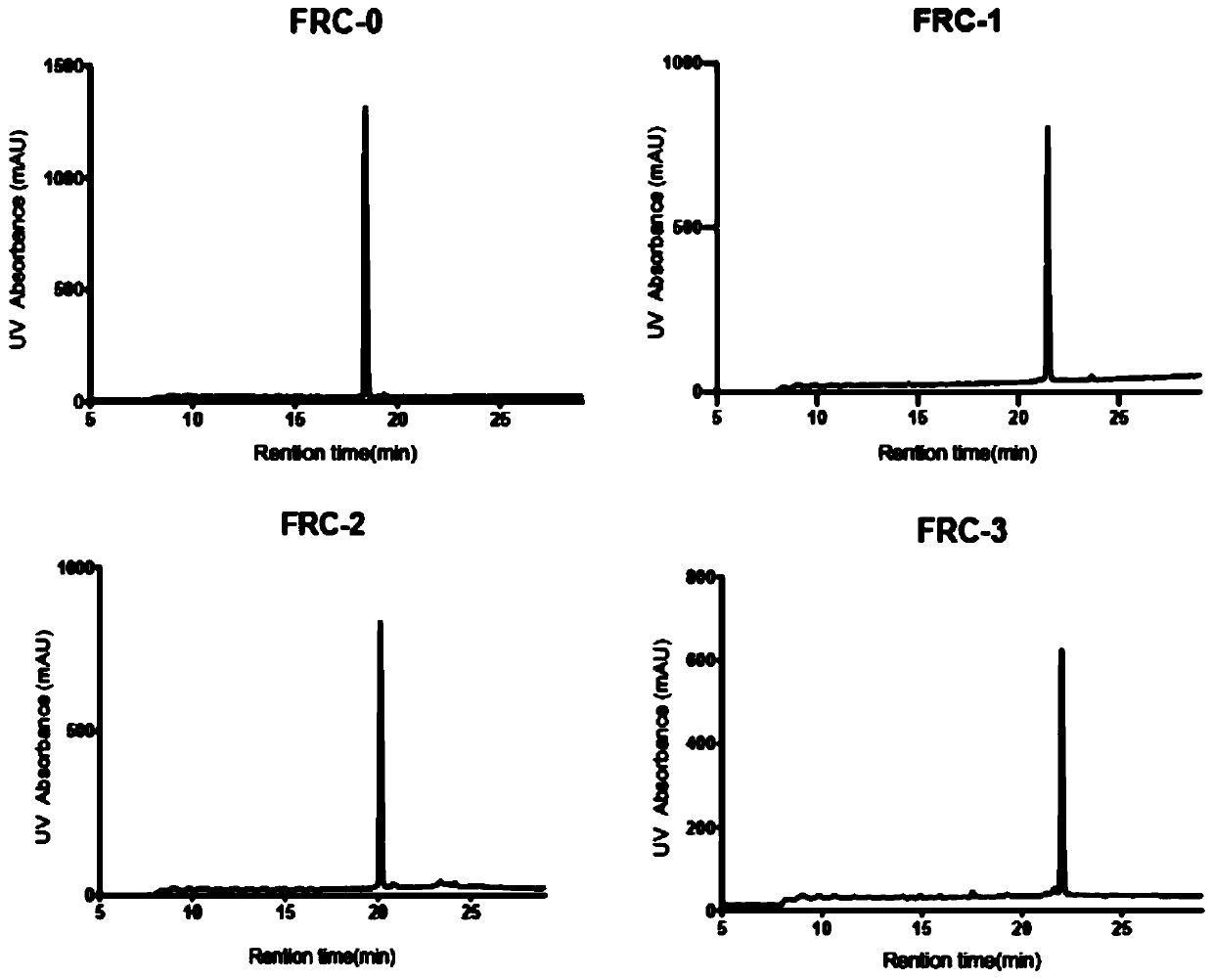
[0087] The product obtained in step 2 of Example 1 was identified by HPLC and structurally analyzed by HR-Q-TOF-MS (high-resolution matrix-assisted laser desorption ionization time-of-flight mass spectrometry), and the chromatographic mobile phase was acetonitrile and water. Mobile phase A is an aqueous solution with a volume fraction of 0.1% TFA, and mobile phase B is an acetonitrile solution with a volume fraction of 0.1% TFA, gradient elution (0-5min, mobile phase B: 5%; 5-30min, mobile phase B: 5%~90%); flow rate 15.0mL·min -1 ; The detection wavelengths are 214nm and 254nm, and the injection volume is 20μl. It is determined that it is consistent with the peak time of the main peak of the crude product, and the purity of the staple peptide prepared by this method is >98% ( Figure 3-4 ). The results were analyzed by HR-ESI-MS mass spectrometer as Figure 5-12 shown.
Embodiment 3
[0088] Example 3 Inhibition of osteoclast differentiation experiment
[0089] TRAP staining assay: Osteoclast formation was quantified by TRAP-positive cells. BMMs were seeded into 96-well plates at a density of 8000 cells per well. After incubation for 24 h, BMMs were incubated with M-CSF at a concentration of 30 ng / ml, RANKL at 50 ng / mL and different concentrations of peptides (0, 20 and 40 μM). Fresh medium was replaced every 2 days. After 5 days of osteoclast induction, wash twice with PBS, fix in 4% paraformaldehyde (pH 7.4) at room temperature for 10 min, and stain with TRAP staining kit. Positive multinucleated osteoclasts were counted under a light microscope, and the percentage of osteoclasts in each well was measured using Image J software.
[0090] see results Figure 13-14 , the staple peptide of the present invention can inhibit the differentiation of osteoclasts, among which FRN-2, FRC-1, FRC-2 and FRC-3 have the most prominent effects, and FRC-2 has the best...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com